Abstract
PURPOSE
To evaluate the efficacy and safety of tucatinib and trastuzumab in patients with previously treated human epidermal growth factor receptor 2–positive (HER2+) metastatic biliary tract cancer (mBTC).
METHODS
SGNTUC-019 (ClinicalTrials.gov identifier: NCT04579380) is an open-label phase II basket study evaluating the efficacy and safety of tucatinib and trastuzumab in patients with HER2-altered solid tumors. In the biliary tract cancer cohort, patients had previously treated HER2 overexpressing or amplified (HER2+) tumors (identified with local testing) with no prior HER2-directed therapy. The primary end point was confirmed objective response rate (cORR) per investigator assessment. Patients were treated on a 21-day cycle with tucatinib (300 mg orally twice daily) and trastuzumab (8 mg/kg intravenously followed by 6 mg/kg every 3 weeks).
RESULTS
Thirty patients were enrolled. As of data cutoff (January 30, 2023), the median duration of follow-up was 10.8 months. The cORR was 46.7% (90% CI, 30.8 to 63.0), with a disease control rate of 76.7% (90% CI, 60.6 to 88.5). The median duration of response and progression-free survival were 6.0 months (90% CI, 5.5 to 6.9) and 5.5 months (90% CI, 3.9 to 8.1), respectively. At data cutoff, 15 patients (50.0%) had died, and the estimated 12-month overall survival rate was 53.6% (90% CI, 36.8 to 67.8). The two most common treatment-emergent adverse events (TEAEs) were pyrexia (43.3%) and diarrhea (40.0%). Grade ≥3 TEAEs were reported in 18 patients (60.0%), with the most common being cholangitis, decreased appetite, and nausea (all 10.0%), which were generally not treatment related. TEAEs led to treatment regimen discontinuation in one patient, and there were no deaths due to TEAEs.
CONCLUSION
Tucatinib combined with trastuzumab had clinically significant antitumor activity and was well tolerated in patients with previously treated HER2+ mBTC.
INTRODUCTION
Biliary tract cancer (BTC) is an aggressive malignancy, with the majority of patients having metastatic or locally advanced disease at diagnosis.1,2 First-line systemic therapy for advanced BTC, including metastatic BTC (mBTC), includes gemcitabine plus cisplatin with or without durvalumab, with a median overall survival (OS) of 12.8 months and 11.5 months, respectively.3 A recent report has also shown that pembrolizumab added to gemcitabine and cisplatin results in a significantly longer OS compared with gemcitabine and cisplatin alone in the first-line setting (12.7 months v 10.9 months).4 However, for patients whose BTC progresses beyond first-line therapy, treatment options are limited and provide modest clinical benefit.5,6 Currently used second-line treatments, such as infusional fluorouracil, leucovorin, and oxaliplatin and S-1, yield objective response rates of 5% and 7.5%, with a median OS of 6.2 months and 6.8 months, respectively.7,8 Therefore, patients with mBTC that progresses on first-line therapy need well-tolerated treatment options with higher efficacy.
CONTEXT
Key Objective
Is tucatinib combined with trastuzumab effective and safe in patients with previously treated human epidermal growth factor receptor 2–positive (HER2+) metastatic biliary tract cancer (mBTC)?
Knowledge Generated
Tucatinib and trastuzumab showed clinical activity in patients with HER2+ mBTC, with a confirmed objective response rate of 46.7%; the treatment regimen also showed a tolerable and manageable safety profile with low rates of treatment-related serious and high-grade adverse events and treatment discontinuations. The exploratory biomarker analyses demonstrated that multiple HER2 testing methods can be used to help identify patients with HER2+ mBTC who may respond to the treatment regimen.
Relevance (A.H. Ko)
-
HER2 represents a viable target for biliary tract cancers. The promising results from this study, while requiring confirmation in larger cohorts, suggest that the combination of tucatinib and trastuzumab may become a useful therapeutic strategy for the subset of patients with HER2+ disease.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Human epidermal growth factor receptor 2 (HER2) overexpression/amplification (HER2-positive [HER2+]) has been identified as an oncogenic driver in multiple malignancies and may be associated with a poorer prognosis.9 HER2-directed treatments have been shown to be efficacious in several HER2+ solid tumors,10-20 leading to their approval for treatment of HER2+ metastatic breast, gastric, and colorectal cancers. HER2 overexpression/amplification is observed in up to 20% of mBTC, with varying rates on the basis of tumor location.21-23 A recent report has shown that 7.9% of mBTC are HER2+.24 HER2 is emerging as an important actionable target in this patient population,23,25-28 as investigational anti-HER2 therapies have demonstrated clinical activity in mBTC with reported objective response rates (ORRs) ranging from 12% to 41.3%.29-34
Tucatinib is an oral tyrosine kinase inhibitor highly selective for HER2.35 Preclinical data have shown that tucatinib and trastuzumab in various HER2+ tumor types results in superior antitumor activity compared with either agent alone.35,36 Consistent with the preclinical data, clinical trials of tucatinib have demonstrated that vertical receptor inhibition of HER2 is highly effective in patients with HER2+ metastatic cancer. The HER2CLIMB trial (ClinicalTrials.gov identifier: NCT02614794) has shown that adding tucatinib to trastuzumab and capecitabine is well tolerated and improves the OS of patients with previously treated HER2+ metastatic breast cancer, with or without brain metastases.14,37-39 In the phase II MOUNTAINEER study (ClinicalTrials.gov identifier: NCT03043313), tucatinib and trastuzumab were well tolerated and highly effective in patients with previously treated HER2+ metastatic colorectal cancer.20 These data suggest that tucatinib in combination with trastuzumab may have clinical activity in other HER2+ solid tumors. Herein, we present the efficacy and safety results of tucatinib and trastuzumab in a cohort of patients with previously treated HER2+ mBTC from the SGNTUC-019 study.
METHODS
Study Overview
SGNTUC-019 (ClinicalTrials.gov identifier: NCT04579380) is an open-label phase II basket study of patients with previously treated, locally advanced, unresectable or metastatic HER2-altered solid tumors. The study was conducted in accordance with regulatory requirements and International Council for Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent. The Protocol (online only; available with the full text of this article) was approved by institutional review boards and ethics committees according to the practice at each participating trial site.
Study Population
The BTC cohort comprises patients with HER2+ mBTC with measurable disease as per RECIST v1.1. Patients must have progressed during or after at least one prior line of systemic therapy or be intolerant of the most recent line of systemic therapy. Patients with an Eastern Cooperative Oncology Group performance status 0 or 1 and adequate baseline cardiac, hepatic, renal, and hematologic function were eligible. Patients previously treated with any systemic anticancer therapy, radiation therapy, major surgery, or experimental agent within 3 weeks of the first dose of study treatment were excluded. In addition, patients must have not received prior HER2-directed therapy. Full inclusion and exclusion criteria are available in the Protocol.
HER2 overexpression or amplification was determined locally using archival or fresh tumor tissue or blood via any of the following methods: (1) immunohistochemistry (IHC; overexpression defined as IHC 3+), (2) in situ hybridization (fluorescence in situ hybridization [FISH] or chromogenic in situ hybridization [CISH], amplification defined as HER2/CEP17 signal ratio ≥2.0 or gene copy number >6), or (3) next-generation sequencing (NGS) amplification.
Procedures
Patients in the BTC cohort were treated with tucatinib 300 mg orally twice daily and trastuzumab 8 mg/kg intravenously then 6 mg/kg every 3 weeks in a 21-day cycle. Disease response to study treatment and the occurrence of disease progression were determined according to RECIST v1.1, as assessed by the investigator. Disease assessments were performed at baseline, every 6 weeks for the first 24 weeks, then every 12 weeks until the occurrence of documented disease progression per RECIST v1.1, death, withdrawal of consent, loss to follow-up, or study closure.
Safety was assessed by the incidence of treatment-emergent adverse events (TEAEs), graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0, recording of concomitant medication, physical examination findings, vital signs, laboratory tests, pregnancy testing, and cardiac function. Cardiac ejection fraction was assessed via echocardiogram or a multigated acquisition scan at screening and every 12 weeks thereafter.
For the exploratory biomarker assessments, central HER2 testing was performed in Clinical Laboratory Improvement Amendments–accredited laboratories. Patients' blood samples and archival or fresh tumor tissue biopsies (if available) were collected during prescreening, screening, or on day 1 of cycle 1. Central HER2 testing was performed using IHC (PATHWAY anti-HER-2 assay [Roche, Tucson, AZ]), FISH (HER2 IQFISH pharmDx assay [Agilent, Singapore, Singapore]), and blood-based NGS assay (Guardant360, Redwood City, CA). IHC and FISH results were evaluated using the ASCO-College of American Pathologists Gastric Scoring criteria.40
Assessments
The primary end point was confirmed objective response rate (cORR), defined as the proportion of patients with confirmed complete response (CR) or partial response according to RECIST v1.1, per investigator assessment. Secondary end points included disease control rate (DCR), duration of response (DOR), progression-free survival (PFS), OS, and safety. The exploratory end points included time to first response, the percent agreement among results from different local and central testing methods of HER2 overexpression/amplification, and cORR of patients who had HER2+ tumors on the basis of different central testing methods.
Statistical Analysis
The BTC cohort aimed to enroll up to 30 patients, a number calculated per the 90% exact CI given a range of expected cORR of 10%-30%. An interim analysis was to be performed when 12 patients were enrolled or two confirmed responses were observed, whichever was earlier. If there were at least two responders observed among the 12 patients, the predictive probability of success would be >20%, indicating that it is possible that the cORR will be higher than the current standard of care once all 30 patients were enrolled and assessed.
All enrolled patients received at least one dose of tucatinib and trastuzumab and were included in the evaluation for efficacy and safety. Two-sided 90% exact CIs for response rates were calculated by using the Clopper-Pearson method. Median PFS and OS were estimated by using the Kaplan-Meier method; the associated 90% CI was calculated on the basis of the complementary log-log transformation. Safety and concordance of local versus central HER2 testing results were assessed by descriptive statistics. The term percent agreement was used instead of concordance in comparing local and central testing results since all patients had HER2+ tumors per local testing assays. All analyses except the biomarker analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC). Biomarker analyses were performed with R, version 4.0.2 (R Core Team and the R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Thirty patients were enrolled in the BTC cohort from June 7, 2021, to May 30, 2022. As of the data cutoff (January 30, 2023), three remain on study treatment, eight are in long-term follow-up, and 19 are off study. One patient discontinued the treatment regimen (both tucatinib and trastuzumab) because of a TEAE, with 25 patients discontinuing study treatment because of progressive disease and one patient because of patient decision. The patient disposition is summarized in Figure 1. The median duration of follow-up was 10.8 months (range, 1.5-17.1).
FIG 1.

Flow diagram of the BTC cohort. BTC, biliary tract cancer; HER2+, human epidermal growth factor receptor 2–positive.
Demographics and baseline characteristics of patients enrolled in the BTC cohort are shown in Table 1. The median age was 68.5 years (range, 33-79). The majority of patients were Asian (76.7%). Most patients (80.0%) had locally advanced or metastatic disease at initial diagnosis, and all patients had a history of metastatic disease. The median number of prior lines of therapy in any setting and in the locally advanced or metastatic setting was 2.0 (range, 1-4) and 1.0 (range, 1-4), respectively. All 30 patients previously received a gemcitabine and cisplatin-containing regimen, and two patients (6.7%) were previously treated with a PD-1 or PD-L1 inhibitor. All enrolled patients had positive HER2 status on the basis of local testing.
TABLE 1.
Patient Demographics and Baseline Characteristics

Efficacy
The cORR per investigator assessment was 46.7% (14 of 30; 90% CI, 30.8 to 63.0; Table 2). The median DOR was 6.0 months (range, 1.4-13.4). The DCR was 76.7% (90% CI, 60.6 to 88.5). The change in tumor size from baseline among the patients with a baseline and at least one postbaseline measurement is shown in Figures 2 and 3. Twenty-one patients (70.0%) had a reduction in the tumor size (Fig 2). The median time to first response was 2.1 months (range, 1.2-4.3; Fig 3).
TABLE 2.
Summary of Responses

FIG 2.
Maximum percentage reduction in the sum of tumor diameters from baseline per investigator assessment and local HER2 testing results for each patient. One patient was excluded because of missing postbaseline measurement. aNumbers refer to the IHC scores. NA indicates a missing evaluable sample, including quality control fails. b+ is defined as amplification. NA indicates a missing evaluable sample, including quality control fails. B, blood-based; CISH, chromogenic in situ hybridization; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; NA, not available; NGS, next-generation sequencing; T, tissue-based.
FIG 3.

Percentage change from baseline in sum of diameter of target lesions per investigator assessment. One patient was excluded because of no postbaseline measurement.
At time of data cutoff, the median PFS was 5.5 months (90% CI, 3.9 to 8.1; Fig 4A), with an estimated 6-month PFS of 49.8% (90% CI, 34.1 to 63.6) and estimated 12-month PFS of 16.1% (90% CI, 5.9 to 30.6). The median OS was 15.5 months (90% CI, 6.5 to 16.7; Fig 4B), with an estimated 6-month OS of 73.0% (90% CI, 56.9% to 83.9%) and 12-month OS of 53.6% (90% CI, 36.8 to 67.8). Fifteen (50.0%) patients had died at data cutoff (Fig 1).
FIG 4.
Kaplan-Meier curves for (A) PFS and (B) OS. Associated 90% CIs were calculated on the basis of the complementary log-log transformation. OS, overall survival; PFS, progression-free survival.
At data cutoff, 27 patients (90.0%) were off treatment, and among these patients, 7 (25.9%) received at least one subsequent anticancer therapy, including three (11.1%) who received a subsequent HER2-directed therapy.
Safety
The median treatment duration with tucatinib was 5.1 months (range, 0.3-16.2) and with trastuzumab was 5.6 months (range, 0.7-16.8). TEAEs were reported in all patients (Table 3; Appendix Table A1, online only). The five most common TEAEs were pyrexia (13 patients [43.3%]), diarrhea (12 [40.0%]), blood creatinine increased, infusion related reaction, and ALT increased (each in eight [26.7%]; Table 3). Eighteen (60.0%) patients had grade ≥3 TEAEs. The most common grade ≥3 events were nausea, decreased appetite, and cholangitis (each in three [10.0%]). The majority of grade ≥3 TEAEs were not related to study treatment, with grade ≥3 tucatinib-related events reported in seven patients (23.3%) and trastuzumab-related events in three (10.0%). Thirteen patients (43.3%) had serious TEAEs, with three (10.0%) related to tucatinib and two (6.7%) related to trastuzumab. Tucatinib was discontinued because of TEAEs in three patients (10.0%), due to cholangitis (grade 3), interstitial lung disease (grade 3), and liver disorder (grade 4). At the time of data cutoff, the events of liver disorder and cholangitis were resolved, and the event of interstitial lung disease was grade 1. One patient (3.3%) discontinued trastuzumab because of interstitial lung disease (grade 3). Tucatinib dose reductions because of TEAEs were reported in six patients (20.0%). No TEAEs resulted in death. All 15 deaths (50.0%) that occurred at the time of the data cutoff date were related to disease progression.
TABLE 3.
Most Common TEAEs

Of the 12 patients with diarrhea, 10 patients had grade ≤2 events, two had grade 3 events, and there were no grade 4 events. No events of diarrhea led to treatment discontinuation. Events of ALT and aspartate aminotransferase increased were reported in eight and six patients, respectively, and two patients had grade 3 events for both.
Agreement Among HER2 Testing Methods and Treatment Response by HER2 Testing Methods
Among the 30 patients in the BTC cohort, 24 patients had tissue samples evaluable for the exploratory analysis comparing results from local versus central HER2 testing using central IHC and FISH (Appendix Fig A1, online only). Twenty-nine patients had evaluable samples for central blood-based NGS (Appendix Fig A1). The percent agreement between local testing and central IHC/FISH, central FISH, and central blood-based NGS results were, respectively, 87.5% (21 of 24; 90% CI, 70.8 to 96.5; Appendix Table A2, online only), 87.5% (21 of 24; 90% CI, 70.8 to 96.5; Appendix Table A3, online only), and 75.9% (22 of 29; 90% CI, 59.4 to 88.1; Appendix Table A4, online only). The percent agreement between different central HER2 testing assays ranged from 82.4% to 100.0% (Appendix Tables A5-A8, online only).
The cORR for patients confirmed as HER2+ by central IHC/FISH was 57.1% (12 of 21; 90% CI, 37.2 to 75.5; Appendix Table A9, online only) and confirmed as HER2+ by FISH was 57.1% (12 of 21; 90% CI, 37.2 to 75.5; Appendix Table A10, online only). The cORR for patients confirmed by blood-based NGS as HER2+ was 63.6% (14 of 22; 90% CI, 43.9% to 80.4%; Appendix Table A11, online only). All patients who responded were HER2+ by central testing. All patients who tested HER2-negative by any central testing method did not have an objective response to the treatment (Appendix Fig A1).
DISCUSSION
Most patients with BTC are diagnosed with locally advanced or metastatic disease and have a poor prognosis.1,2,9 The median OS for patients with mBTC on first-line therapy is approximately 1 year and for second-line therapy approximately 6 months.3,5-8 Current second-line options for patients with mBTC are limited and yield modest benefit; hence, effective treatment options are needed.
BTC is a heterogeneous group of rare diseases with varied actionable molecular alterations (eg, FGFR2 and IDH1),41 and recent reports have shown that several molecular agents targeting specific genomic alterations result in antitumor activity in patients with mBTC.42 HER2 is a validated target for HER2+ metastatic breast, gastric, and colorectal cancers,10-20 and results from studies with HER2-directed agents also suggest activity in patients with HER2+ mBTC.29-34 Previous studies have demonstrated meaningful antitumor activity treating HER2+ mBTC with HER2-directed agents (ORR of 12%-41.3%).29-34 The results presented in this study indicate that tucatinib and trastuzumab appear to be effective in patients with previously treated HER2+ mBTC, and clinical activity was observed in patients with IHC 3+ or 2+ tumors. Patients treated with the combination had a cORR of 46.7%, which, to our knowledge, is one of the highest reported response rates among investigational HER2-directed therapies for mBTC to date. Responses to treatment with tucatinib and trastuzumab were rapid and durable, with a median time to first response of 2.1 months and a median DOR of 6.0 months. The majority of all enrolled patients experienced a reduction in tumor size, with one patient achieving a CR. Of note, the treatment regimen resulted in a median PFS of 5.5 months (90% CI, 3.9 to 8.1) and a median OS of 15.5 months (90% CI, 6.5 to 16.7). Because of the short follow-up, the median OS should be interpreted with caution. These data support the meaningful clinical activity of tucatinib and trastuzumab in patients with HER2+ mBTC.
The combination of tucatinib and trastuzumab was well tolerated and consistent with the previously reported safety profile of the regimen.20 Chemotherapy-related toxicities are of considerable concern for patients with mBTC on first- and second-line therapies. Grade ≥3 TEAEs are reported in approximately 69%-78% of patients, highlighting a need for better tolerated, chemotherapy-free treatment options.3,7 Tucatinib and trastuzumab were well tolerated with a low incidence of treatment-related events; only 23.3% and 10.0% of the patients had grade ≥3 TEAEs related to tucatinib and trastuzumab, respectively. In addition, only one patient discontinued the study regimen because of TEAEs. Diarrhea was reported in 40.0% of patients, but most events were grade 1. Given the primary tumor location in the biliary tract and the propensity for the tumor to spread locally, the increase in aminotransferase levels may be explained by complications associated with the primary site of the tumor, such as cholangitis.43
There is currently no consistent HER2 testing guideline for BTC.44 A more extensive application of HER2 testing may help with informing treatment decisions, and patients with mBTC with molecular alterations may have improved outcomes when treated with a tailored targeted therapy.41,45,46 A comparative analysis of results from local and central testing showed a high level of agreement between central and local and between central HER2 status determination. In addition, cORR for patients whose samples tested positive for HER2 centrally (IHC/FISH, 57.1%; FISH only, 57.1%; NGS, 63.6%) was similar to the cORR reported in the overall cohort (46.7%). The percent agreement and cORR data suggest that in clinical setting, various HER2 testing modalities can reliably be used to identify HER2+ patients with mBTC who may respond to tucatinib and trastuzumab. No responses were seen in patients who tested negative by the central blood-based NGS assay, suggesting clinical utility of this platform in HER2+ mBTC.
This analysis is limited by a small cohort, short follow-up, lack of control group, and absence of independent central radiology review. Despite the absence of a control arm, the encouraging results from this analysis support that HER2 is an actionable biomarker for HER2+ mBTC, justifying further investigation of HER2-directed agents in this tumor type. Most patients enrolled in the BTC cohort were Asian, which is consistent with the global statistics for BTC; Japan and South Korea are known to have some of the highest incidences of the disease.47 Additionally, to contextualize our data, we have referenced previous studies with other HER2-directed agents in similar patient populations while acknowledging the limitations of cross-trial comparisons. Finally, the patients were enrolled on the basis of various local HER2 testing modalities that could be performed on archival tissues, and the assessments were heterogeneous because of lack of standardized testing guidelines for BTC. Local and central HER2 test results had a high level of agreement. These results highlight the importance of HER2 testing for patients with mBTC to optimize clinical treatment, and the testing methods used in this analysis could serve as a framework for future studies involving patients with HER2+ mBTC or other types of tumors.
The results from the BTC cohort of SGNTUC-019 study further validate HER2 as an actionable biomarker in mBTC, and additional investigations of HER2-directed agents are warranted in patients with HER2+ mBTC. The combination of tucatinib and trastuzumab in this analysis demonstrated clinically meaningful activity and favorable tolerability for patients with HER2+ mBTC, a population with historically poor outcomes.
ACKNOWLEDGMENT
The authors thank the patients who participated in this trial, their families, and the investigators and staff at SGNTUC-019 clinical sites. Dr Nakamura thanks the SCRUM-Japan MONSTAR-SCREEN alliance for contributing to the patient recruitment at the Japanese study sites. The authors also thank Mark Bieda, PhD, Amanda Drees, PharmD, and Keith Earley, PhD, of Seagen Inc (Bothell, WA) for critical review of the data and the manuscript and Matthew Blahna, PhD, and Mariah van Waes, PharmD, of Seagen Inc for critical review of the manuscript. Finally, the authors thank the entire SGNTUC-019 study team for support. Kavya Kalachaveedu, PharmD, of MMS Holdings (Canton, MI) and Irene Park, PhD, of Seagen Inc provided medical writing and editorial support with funding from Seagen Inc, in accordance with Good Publication Practice guidelines. F. Hoffmann-La Roche, San Francisco, CA, provided trastuzumab for the study.
APPENDIX
TABLE A1.
Summary of Safety Events

TABLE A2.
Percent Agreement of HER2 Status for Local Versus Central IHC/FISH Testing
TABLE A3.
Percent Agreement of HER2 Status for Local Versus Central FISH Testing
TABLE A4.
Percent Agreement of HER2 Status for Local Versus Central Blood-Based NGS Testing
TABLE A5.
Percent Agreement of HER2 Status Between Central IHC/FISH and Blood-Based NGS Testing

TABLE A6.
Percent Agreement of HER2 Status Between Central FISH and Blood-Based NGS Testing
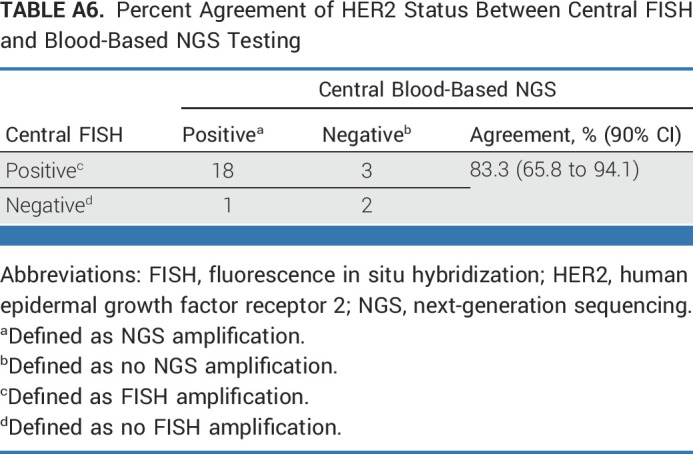
TABLE A7.
Percent Agreement of HER2 Status Between Central IHC and FISH Testing

TABLE A8.
Percent Agreement of HER2 Status Between Central IHC and Blood-Based NGS Testing

TABLE A9.
HER2 Status by Central IHC/FISH Testing Versus Treatment Response

TABLE A10.
HER2 Status by Central FISH Testing Versus Treatment Response

TABLE A11.
HER2 Status by Central Blood-Based NGS Testing Versus Treatment Response

FIG A1.
Summary of HER2 testing results and best overall response. Each column represents one patient. One patient was not included because of missing postbaseline measurement. The one excluded patient had IHC 3+ tumor per local testing (NA for FISH/CISH and NGS). Per central testing, this patient had IHC 1+ and no amplification per FISH and blood-based NGS. aNumbers refer to the IHC scores. NA indicates a missing evaluable sample, including quality control fails. bNo scoring criteria were specified for local IHC. c+ is defined as amplification. NA indicates a missing evaluable sample, including quality control fails. dEvaluated by using the ASCO-College of American Pathologists Gastric Scoring criteria. e+ is defined as amplification. – is defined as no amplification. NA indicates a missing evaluable sample, including quality control fails. B, blood-based; CISH, chromogenic in situ hybridization; CR, complete response; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NA, not available; NGS, next-generation sequencing; PD, progressive disease; PR, partial response; SD, stable disease; T, tissue-based.
Yoshiaki Nakamura
Honoraria: Chugai Pharma, Guardant Health AMEA, Merck
Research Funding: Taiho Pharmaceutical (Inst), Guardant Health (Inst), Genomedia (Inst), Chugai Pharma (Inst), Seagen (Inst), Roche (Inst)
Nobumasa Mizuno
Honoraria: Yakult Honsha, AstraZeneca, Novartis, Fujifilm, Taiho Pharmaceutical
Research Funding: MSD (Inst), Incyte (Inst), Ono Pharmaceutical (Inst), Seagen (Inst), Novartis (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Takeda, Chugai Pharma, Lilly Japan, Otsuka
Jean-Luc Canon
Honoraria: AstraZeneca/Daiichi Sankyo, Lilly, Seagen
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo, Lilly, Seagen, Sanofi
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb/Pfizer (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo/Astra Zeneca, Roche/Genentech
Matthew D. Galsky
Stock and Other Ownership Interests: Rappta Therapeutics
Consulting or Advisory Role: BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas Pharma, Genentech, Bristol Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seagen, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics, Basilea, Urogen Pharma, Infinity Pharmaceuticals, Gilead Sciences, Silverback Therapeutics, AbbVie
Research Funding: Janssen Oncology (Inst), Dendreon (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Methods and compositions for treating cancer and related methods. Mount Sinai School Of Medicine July 2012 Application number: 20120322792
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Mersana (Inst), AstraZeneca (Inst), Novartis (Inst), Seagen (Inst), ITeos Therapeutics (Inst), Janssen (Inst), Loxo (Inst), Relay Therapeutics (Inst), Greenwich LifeSciences (Inst), Orum Therapeutics (Inst), Ellipses Pharma (Inst), Olema Pharmaceuticals (Inst), Stemline Therapeutics (Inst), Tubulis GmbH (Inst), Verascity Science (Inst), Theratechnologies (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros Pharmaceuticals (Inst), Clovis Oncology (Inst), CytomX Therapeutics (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro Biopharma (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Black Diamond Therapeutics (Inst), Orinove (Inst), Molecular Templates (Inst), Seagen (Inst), Compugen (Inst), G1 Therapeutics (Inst), Karyopharm Therapeutics (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), PharmaMar (Inst), Olema Pharmaceuticals (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), Akeso Biopharma (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Jacobio (Inst), Accutar Biotech (Inst), Artios (Inst), Bliss Biopharmaceutical (Inst), Cascadian Therapeutics (Inst), Dantari (Inst), Duality Biologics (Inst), Elucida Oncology (Inst), Infinity Pharmaceuticals (Inst), Relay Therapeutics (Inst), Tolmar (Inst), Torque (Inst), BeiGene (Inst), Context Therapeutics (Inst), K-Group Beta (Inst), Kind Pharmaceuticals (Inst), Loxo (Inst), Oncothyreon (Inst), Orum Therapeutics (Inst), Prelude Therapeutics (Inst), ProfoundBio (Inst), Cullinan Oncology (Inst)
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono, Amgen, Daiichi Sankyo/UCB Japan, Guardant Health, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Janssen
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co, Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), PAREXEL (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Sysmex (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Guy Jerusalem
Honoraria: Novartis, Roche, Lilly, Pfizer, Bristol Myers Squibb, AstraZeneca, Daiichy Sankyo, Seagen
Consulting or Advisory Role: Novartis, Roche, Pfizer, Bristol Myers Squibb, Lilly, AstraZeneca, Daiichi Sankyo, Seagen
Travel, Accommodations, Expenses: Novartis, Roche, Pfizer, Lilly, Amgen, BMS, AstraZeneca
Keun-Wook Lee
Honoraria: JW Pharmaceutical, Sanofi/Aventis, Astellas Pharma, Bayer, Daiichi Sankyo
Consulting or Advisory Role: Daiichi Sankyo, MSD, Vifor Pharma, Metafines, Ono Pharmaceutical
Research Funding: Macrogenics (Inst), MSD (Inst), Ono Pharmaceutical (Inst), GC Pharma (Inst), AstraZeneca/MedImmune (Inst), LSK BioPharma (Inst), Merck KGaA (Inst), Pharmacyclics (Inst), Pfizer (Inst), ALX Oncology (Inst), Zymeworks (Inst), BeiGene (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), ABL Bio (Inst), Y-Biologics (Inst), Oncologie (Inst), Seagen (Inst), Bolt Biotherapeutics (Inst), Trishula Therapeutics (Inst), InventisBio (Inst), Leap Therapeutics (Inst), Astellas Pharma (Inst), MedPacto (Inst), Ildong Pharmaceutical (Inst), Roche (Inst), Amgen (Inst), Genome & Company (Inst), Arcus Biosciences (Inst)
Lionel Kankeu Fonkoua
Honoraria: Exelixis (Inst), Incyte (Inst)
Consulting or Advisory Role: Incyte (Inst), Exelixis (Inst)
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals, Acrivon Therapeutics, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, TESARO/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
Danny Nguyen
Stock and Other Ownership Interests: Intuitive Surgical, Teladoc
Consulting or Advisory Role: Janssen Oncology
Other Relationship: Takeda, Novartis
Uncompensated Relationships: Takeda, Novartis
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics, Yuhan, Arcus Biosciences, IQVIA
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Alicia Okines
Stock and Other Ownership Interests: AstraZeneca
Honoraria: Gilead Sciences, Seagen, AstraZeneca
Consulting or Advisory Role: Roche/Genentech, Seagen, AstraZeneca/Daiichi Sankyo, Pfizer
Speakers' Bureau: Seagen, Pfizer, Lilly, AstraZeneca, Gilead Sciences, Eisai
Research Funding: Pfizer (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: LEO Pharma, AstraZeneca, Daiichi Sankyo Europe GmbH, Lilly, Roche
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Agenus, Eisai, Genelux, GlaxoSmithKline, Regeneron, Elevar Therapeutics, Novartis, Seagen, BBI Healthcare, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche, Corcept Therapeutics, Imvax, Jazz Pharmaceuticals, Merck, Verastem, Umoja Biopharma, OncoC4, Mersana, DualityBio
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seagen (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology, Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), NovoCure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Paula Pohlmann
Leadership: Immunonet BioSciences
Stock and Other Ownership Interests: Immunonet BioSciences
Honoraria: Dava Oncology, OncLive/MJH Life Sciences, Frontiers Media
Consulting or Advisory Role: Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, Heron, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, Juniper Pharmaceuticals, Bolt Biotherapeutics, AbbVie
Speakers' Bureau: Genentech/Roche
Research Funding: Genentech/Roche (Inst), Fabre-Kramer (Inst), Advanced Cancer Therapeutics (Inst), Caris Centers of Excellence (Inst), Pfizer (Inst), Pieris Pharmaceuticals (Inst), Cascadian Therapeutics (Inst), Bolt Biotherapeutics (Inst), Byondis (Inst), Seagen (Inst)
Patents, Royalties, Other Intellectual Property: United States Patent no. 8,486,413, United States Patent no. 8,501,417, United States Patent no. 9,023,362, United States Patent no. 9,745,377, Patent application
Uncompensated Relationships: Seagen, Pfizer
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Kazuki Sudo
Honoraria: AstraZeneca, Pfizer, Eisai, Nihon Medi-Physics
Research Funding: NanoCarrier, Daiichi Sankyo, AstraZeneca, Pfizer, Amgen, PRA Health Sciences, Takeda, Merck
Shunji Takahashi
Honoraria: Daiichi Sankyo, Eisai, Bayer, Taiho Pharmaceutical, MSD, Chugai Pharma, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan
Consulting or Advisory Role: Bayer
Research Funding: Daiichi Sankyo (Inst), Sanofi (Inst), Eisai (Inst), Bayer (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Chugai Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Pfizer/EMD Serono (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Astellas Pharma (Inst)
Cedric Van Marcke
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Bristol Myers Squibb, Amgen, Roche Belgium, Pfizer, Roche, Merck, MSD Oncology, Digicore
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Oncternal Therapeutics, AADi
Research Funding: Dendreon (Inst), Merck (Inst), Seagen (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst), Tyra Biosciences (Inst)
Roman Groisberg
Employment: Merck
Stock and Other Ownership Interests: Merck
Jorge Ramos
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Sherry Tan
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Travel, Accommodations, Expenses: Seagen
Thomas E. Stinchcombe
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Janssen Oncology, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo/Astra Zeneca, Takeda, Eisai/H3 Biomedicine, G1 Therapeutics, Spectrum Pharmaceuticals, Gilead Sciences, AstraZeneca, Coherus Biosciences
Research Funding: AstraZeneca (Inst), Seagen (Inst), Mirati Therapeutics (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
Tanios Bekaii-Saab
Consulting or Advisory Role: Amgen (Inst), Ipsen (Inst), Lilly (Inst), Bayer (Inst), Roche/Genentech (Inst), AbbVie, Incyte (Inst), Immuneering, Seagen (Inst), Pfizer (Inst), Boehringer Ingelheim, Janssen, Eisai, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine, Arcus Biosciences (Inst), Stemline Therapeutics, Kanaph Therapeutics, Deciphera, Illumina, Caladrius Biosciences, Zai Lab
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck (Inst), AstraZeneca, Lilly, Pancreatic Cancer Action Network, FibroGen, Suzhou Kintor Pharmaceuticals, 1Globe Health Institute, Imugene, Xilis, Replimune, Sun Biopharma, UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636276
No other potential conflicts of interest were reported.
SUPPORT
Supported by Seagen Inc (Bothell, WA) in collaboration with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc (Rahway, NJ).
CLINICAL TRIAL INFORMATION
NCT04579380 (SGNTUC-019)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.00606.
AUTHOR CONTRIBUTIONS
Conception and design: Yoshiaki Nakamura, Bradley J. Monk, Do-Youn Oh, Alicia Okines, Paula Pohlmann, Martin Reck, Jorge Ramos, Thomas E. Stinchcombe, Tanios Bekaii-Saab
Provision of study materials or patients: Nobumasa Mizuno, Yu Sunakawa, Jean-Luc Canon, Matthew D. Galsky, Erika Hamilton, Hidetoshi Hayashi, Guy Jerusalem, Seung Tae Kim, Lionel Kankeu Fonkoua, Bradley J. Monk, Do-Youn Oh, Alicia Okines, Martin Reck, Cedric Van Marcke, Evan Y. Yu, Roman Groisberg, Thomas E. Stinchcombe, Tanios Bekaii-Saab
Collection and assembly of data: Yoshiaki Nakamura, Nobumasa Mizuno, Yu Sunakawa, Jean-Luc Canon, Matthew D. Galsky, Erika Hamilton, Hidetoshi Hayashi, Seung Tae Kim, Keun-Wook Lee, Bradley J. Monk, Danny Nguyen, Do-Youn Oh, Alicia Okines, Sang Joon Shin, Kazuki Sudo, Shunji Takahashi, Cedric Van Marcke, Evan Y. Yu, Roman Groisberg, Jorge Ramos, Thomas E. Stinchcombe, Tanios Bekaii-Saab
Data analysis and interpretation: Yoshiaki Nakamura, Nobumasa Mizuno, Jean-Luc Canon, Matthew D. Galsky, Guy Jerusalem, Lionel Kankeu Fonkoua, Bradley J. Monk, Danny Nguyen, Do-Youn Oh, Alicia Okines, Paula Pohlmann, Martin Reck, Kazuki Sudo, Shunji Takahashi, Evan Y. Yu, Roman Groisberg, Jorge Ramos, Sherry Tan, Thomas E. Stinchcombe, Tanios Bekaii-Saab
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tucatinib and Trastuzumab for Previously Treated Human Epidermal Growth Factor Receptor 2–Positive Metastatic Biliary Tract Cancer (SGNTUC‐019): A Phase II Basket Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yoshiaki Nakamura
Honoraria: Chugai Pharma, Guardant Health AMEA, Merck
Research Funding: Taiho Pharmaceutical (Inst), Guardant Health (Inst), Genomedia (Inst), Chugai Pharma (Inst), Seagen (Inst), Roche (Inst)
Nobumasa Mizuno
Honoraria: Yakult Honsha, AstraZeneca, Novartis, Fujifilm, Taiho Pharmaceutical
Research Funding: MSD (Inst), Incyte (Inst), Ono Pharmaceutical (Inst), Seagen (Inst), Novartis (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Takeda, Chugai Pharma, Lilly Japan, Otsuka
Jean-Luc Canon
Honoraria: AstraZeneca/Daiichi Sankyo, Lilly, Seagen
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo, Lilly, Seagen, Sanofi
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb/Pfizer (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo/Astra Zeneca, Roche/Genentech
Matthew D. Galsky
Stock and Other Ownership Interests: Rappta Therapeutics
Consulting or Advisory Role: BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas Pharma, Genentech, Bristol Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seagen, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics, Basilea, Urogen Pharma, Infinity Pharmaceuticals, Gilead Sciences, Silverback Therapeutics, AbbVie
Research Funding: Janssen Oncology (Inst), Dendreon (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Methods and compositions for treating cancer and related methods. Mount Sinai School Of Medicine July 2012 Application number: 20120322792
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Mersana (Inst), AstraZeneca (Inst), Novartis (Inst), Seagen (Inst), ITeos Therapeutics (Inst), Janssen (Inst), Loxo (Inst), Relay Therapeutics (Inst), Greenwich LifeSciences (Inst), Orum Therapeutics (Inst), Ellipses Pharma (Inst), Olema Pharmaceuticals (Inst), Stemline Therapeutics (Inst), Tubulis GmbH (Inst), Verascity Science (Inst), Theratechnologies (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros Pharmaceuticals (Inst), Clovis Oncology (Inst), CytomX Therapeutics (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro Biopharma (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Black Diamond Therapeutics (Inst), Orinove (Inst), Molecular Templates (Inst), Seagen (Inst), Compugen (Inst), G1 Therapeutics (Inst), Karyopharm Therapeutics (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), PharmaMar (Inst), Olema Pharmaceuticals (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), Akeso Biopharma (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Jacobio (Inst), Accutar Biotech (Inst), Artios (Inst), Bliss Biopharmaceutical (Inst), Cascadian Therapeutics (Inst), Dantari (Inst), Duality Biologics (Inst), Elucida Oncology (Inst), Infinity Pharmaceuticals (Inst), Relay Therapeutics (Inst), Tolmar (Inst), Torque (Inst), BeiGene (Inst), Context Therapeutics (Inst), K-Group Beta (Inst), Kind Pharmaceuticals (Inst), Loxo (Inst), Oncothyreon (Inst), Orum Therapeutics (Inst), Prelude Therapeutics (Inst), ProfoundBio (Inst), Cullinan Oncology (Inst)
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono, Amgen, Daiichi Sankyo/UCB Japan, Guardant Health, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Janssen
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co, Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), PAREXEL (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Sysmex (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Guy Jerusalem
Honoraria: Novartis, Roche, Lilly, Pfizer, Bristol Myers Squibb, AstraZeneca, Daiichy Sankyo, Seagen
Consulting or Advisory Role: Novartis, Roche, Pfizer, Bristol Myers Squibb, Lilly, AstraZeneca, Daiichi Sankyo, Seagen
Travel, Accommodations, Expenses: Novartis, Roche, Pfizer, Lilly, Amgen, BMS, AstraZeneca
Keun-Wook Lee
Honoraria: JW Pharmaceutical, Sanofi/Aventis, Astellas Pharma, Bayer, Daiichi Sankyo
Consulting or Advisory Role: Daiichi Sankyo, MSD, Vifor Pharma, Metafines, Ono Pharmaceutical
Research Funding: Macrogenics (Inst), MSD (Inst), Ono Pharmaceutical (Inst), GC Pharma (Inst), AstraZeneca/MedImmune (Inst), LSK BioPharma (Inst), Merck KGaA (Inst), Pharmacyclics (Inst), Pfizer (Inst), ALX Oncology (Inst), Zymeworks (Inst), BeiGene (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), ABL Bio (Inst), Y-Biologics (Inst), Oncologie (Inst), Seagen (Inst), Bolt Biotherapeutics (Inst), Trishula Therapeutics (Inst), InventisBio (Inst), Leap Therapeutics (Inst), Astellas Pharma (Inst), MedPacto (Inst), Ildong Pharmaceutical (Inst), Roche (Inst), Amgen (Inst), Genome & Company (Inst), Arcus Biosciences (Inst)
Lionel Kankeu Fonkoua
Honoraria: Exelixis (Inst), Incyte (Inst)
Consulting or Advisory Role: Incyte (Inst), Exelixis (Inst)
Bradley J. Monk
Leadership: US Oncology
Honoraria: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, GOG Foundation, Elevar Therapeutics, Novocure, Gradalis, Karyopharm Therapeutics, Bayer, EMD Serono/Merck, Sorrento Therapeutics, US Oncology, Myriad Pharmaceuticals, Novartis, OncoC4, Pieris Pharmaceuticals, Acrivon Therapeutics, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Consulting or Advisory Role: Agenus, Akeso Biopharma, Amgen, Aravive, AstraZeneca, Clovis Oncology, Eisai, Genmab/Seattle Genetics, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Myriad Pharmaceuticals, Pfizer, Puma Biotechnology, Regeneron, Roche/Genentech, TESARO/GSK, Vascular Biogenics, Gradalis, Karyopharm Therapeutics, Sorrento Therapeutics, Novocure, Bayer, Elevar Therapeutics, EMD Serono/Merck, US Oncology, Novartis, Pieris Pharmaceuticals, OncoC4, Adaptimmune, HenRui, Laekna Health Care, Panavance Therapeutics, Verastem, Zentalis
Speakers' Bureau: Roche/Genentech, AstraZeneca, Clovis Oncology, Eisai, TESARO/GSK, Merck
Research Funding: Novartis (Inst), Amgen (Inst), Genentech (Inst), Lilly (Inst), Janssen (Inst), Array BioPharma (Inst), Tesaro (Inst), Morphotek (Inst), Pfizer (Inst), Advaxis (Inst), AstraZeneca (Inst), Immunogen (Inst), Regeneron (Inst), Nucana (Inst)
Danny Nguyen
Stock and Other Ownership Interests: Intuitive Surgical, Teladoc
Consulting or Advisory Role: Janssen Oncology
Other Relationship: Takeda, Novartis
Uncompensated Relationships: Takeda, Novartis
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics, Yuhan, Arcus Biosciences, IQVIA
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Alicia Okines
Stock and Other Ownership Interests: AstraZeneca
Honoraria: Gilead Sciences, Seagen, AstraZeneca
Consulting or Advisory Role: Roche/Genentech, Seagen, AstraZeneca/Daiichi Sankyo, Pfizer
Speakers' Bureau: Seagen, Pfizer, Lilly, AstraZeneca, Gilead Sciences, Eisai
Research Funding: Pfizer (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: LEO Pharma, AstraZeneca, Daiichi Sankyo Europe GmbH, Lilly, Roche
David M. O'Malley
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Novocure, Genentech/Roche, Immunogen, GOG Foundation, Agenus, Eisai, Genelux, GlaxoSmithKline, Regeneron, Elevar Therapeutics, Novartis, Seagen, BBI Healthcare, Toray Industries, Takeda, InxMed, Celsion, Arcus Biosciences, Sutro Biopharma, Atossa Therapeutics, Laekna Therapeutics, Onconova Therapeutics, VBL Therapeutics, Vincerx Pharma, Adaptimmune, Roche, Corcept Therapeutics, Imvax, Jazz Pharmaceuticals, Merck, Verastem, Umoja Biopharma, OncoC4, Mersana, DualityBio
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), TRACON Pharma (Inst), Genmab (Inst), Seagen (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst), Mersana (Inst), Eisai (Inst), BBI Healthcare (Inst), Sumitomo Dainippon Pharma Oncology, Inc (Inst), Acerta Pharma (Inst), Advaxis (Inst), Arcus Biosciences (Inst), Deciphera (Inst), Exelixis (Inst), Roche (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Ludwig Institute for Cancer Research (Inst), Novartis (Inst), NovoCure (Inst), OncoQuest (Inst), BeiGene (Inst), Pfizer (Inst), Precision Therapeutics (Inst), Sanofi (Inst), Sutro Biopharma (Inst), GlaxoSmithKline (Inst), Verastem (Inst)
Paula Pohlmann
Leadership: Immunonet BioSciences
Stock and Other Ownership Interests: Immunonet BioSciences
Honoraria: Dava Oncology, OncLive/MJH Life Sciences, Frontiers Media
Consulting or Advisory Role: Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, Heron, Puma Biotechnology, Sirtex Medical, Caris Life Sciences, Juniper Pharmaceuticals, Bolt Biotherapeutics, AbbVie
Speakers' Bureau: Genentech/Roche
Research Funding: Genentech/Roche (Inst), Fabre-Kramer (Inst), Advanced Cancer Therapeutics (Inst), Caris Centers of Excellence (Inst), Pfizer (Inst), Pieris Pharmaceuticals (Inst), Cascadian Therapeutics (Inst), Bolt Biotherapeutics (Inst), Byondis (Inst), Seagen (Inst)
Patents, Royalties, Other Intellectual Property: United States Patent no. 8,486,413, United States Patent no. 8,501,417, United States Patent no. 9,023,362, United States Patent no. 9,745,377, Patent application
Uncompensated Relationships: Seagen, Pfizer
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Kazuki Sudo
Honoraria: AstraZeneca, Pfizer, Eisai, Nihon Medi-Physics
Research Funding: NanoCarrier, Daiichi Sankyo, AstraZeneca, Pfizer, Amgen, PRA Health Sciences, Takeda, Merck
Shunji Takahashi
Honoraria: Daiichi Sankyo, Eisai, Bayer, Taiho Pharmaceutical, MSD, Chugai Pharma, Bristol Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan
Consulting or Advisory Role: Bayer
Research Funding: Daiichi Sankyo (Inst), Sanofi (Inst), Eisai (Inst), Bayer (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), Novartis (Inst), Chugai Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Pfizer/EMD Serono (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Astellas Pharma (Inst)
Cedric Van Marcke
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Bristol Myers Squibb, Amgen, Roche Belgium, Pfizer, Roche, Merck, MSD Oncology, Digicore
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Oncternal Therapeutics, AADi
Research Funding: Dendreon (Inst), Merck (Inst), Seagen (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst), Tyra Biosciences (Inst)
Roman Groisberg
Employment: Merck
Stock and Other Ownership Interests: Merck
Jorge Ramos
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Sherry Tan
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Travel, Accommodations, Expenses: Seagen
Thomas E. Stinchcombe
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Janssen Oncology, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo/Astra Zeneca, Takeda, Eisai/H3 Biomedicine, G1 Therapeutics, Spectrum Pharmaceuticals, Gilead Sciences, AstraZeneca, Coherus Biosciences
Research Funding: AstraZeneca (Inst), Seagen (Inst), Mirati Therapeutics (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
Tanios Bekaii-Saab
Consulting or Advisory Role: Amgen (Inst), Ipsen (Inst), Lilly (Inst), Bayer (Inst), Roche/Genentech (Inst), AbbVie, Incyte (Inst), Immuneering, Seagen (Inst), Pfizer (Inst), Boehringer Ingelheim, Janssen, Eisai, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine, Arcus Biosciences (Inst), Stemline Therapeutics, Kanaph Therapeutics, Deciphera, Illumina, Caladrius Biosciences, Zai Lab
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck (Inst), AstraZeneca, Lilly, Pancreatic Cancer Action Network, FibroGen, Suzhou Kintor Pharmaceuticals, 1Globe Health Institute, Imugene, Xilis, Replimune, Sun Biopharma, UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636276
No other potential conflicts of interest were reported.
REFERENCES
- 1.Valle JW: Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol 21:vii345-vii348, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Fong Y, DeMatteo RP, et al. : Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234:507-519, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh D-Y, Ruth He A, Qin S, et al. : Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 4.Kelley RK, Ueno M, Yoo C, et al. : Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401:1853-1865, 2023 [DOI] [PubMed] [Google Scholar]
- 5.Lamarca A, Hubner RA, David Ryder W, et al. : Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann Oncol 25:2328-2338, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Khankhel ZS, Goring S, Bobiak S, et al. : Second-line treatments in advanced biliary tract cancer: Systematic literature review of efficacy, effectiveness and safety. Future Oncol 18:2321-2338, 2022 [DOI] [PubMed] [Google Scholar]
- 7.Lamarca A, Palmer DH, Wasan HS, et al. : Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol 22:690-701, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki E, Ikeda M, Okusaka T, et al. : A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 71:1141-1146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivaldi C, Fornaro L, Ugolini C, et al. : HER2 overexpression as a poor prognostic determinant in resected biliary tract cancer. Oncologist 25:886-893, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Cortes J, Kim SB, et al. : Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109-119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Okamoto W, Kato T, et al. : Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat Med 27:1899-1903, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy RK, Loi S, Okines A, et al. : Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597-609, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Modi S, Saura C, Yamashita T, et al. : Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610-621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shitara K, Bang YJ, Iwasa S, et al. : Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419-2430, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Siena S, Di Bartolomeo M, Raghav K, et al. : Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol 22:779-789, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Saura C, Oliveira M, Feng YH, et al. : Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Phase III NALA trial. J Clin Oncol 38:3138-3149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krop IE, Kim SB, Martin AG, et al. : Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 18:743-754, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Strickler JH, Cercek A, Siena S, et al. : Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol 24:496-508, 2023 [DOI] [PubMed] [Google Scholar]
- 21.Galdy S, Lamarca A, McNamara MG, et al. : HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev 36:141-157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valle JW, Lamarca A, Goyal L, et al. : New horizons for precision medicine in biliary tract cancers. Cancer Discov 7:943-962, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam AR, Kim JW, Cha Y, et al. : Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 7:58007-58021, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouattour M, Valle JW, Vogel A, et al. : Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. 2023 ASCO Annual Meeting, Chicago, IL, June 2-6, 2023 2023 (poster 531)
- 25.Woods E, Le D, Jakka BK, et al. : Changing landscape of systemic therapy in biliary tract cancer. Cancers (Basel) 14:2137, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javle M, Churi C, Kang HC, et al. : HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 8:58, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorscher S: Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Manag Res 9:1-3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondaca S, Razavi P, Xu C, et al. : Genomic characterization of ERBB2-driven biliary cancer and a case of response to ado-trastuzumab emtansine. JCO Precis Oncol 3:1-8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javle M, Borad MJ, Azad NS, et al. : Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 22:1290-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Meric-Bernstam F, Beeram M, Hamilton E, et al. : Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol 23:1558-1570, 2022 [DOI] [PubMed] [Google Scholar]
- 31.Ohba A, Morizane C, Kawamoto Y, et al. : Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J Clin Oncol 40, 2022. (suppl 16; abstr 4006) [DOI] [PubMed] [Google Scholar]
- 32.Lee CK, Chon HJ, Cheon J, et al. : Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: A multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol 8:56-65, 2023 [DOI] [PubMed] [Google Scholar]
- 33.Harding JJ, Piha-Paul SA, Shah RH, et al. : Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nat Commun 14:630, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding JJ, Fan J, Oh DY, et al. : Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): A multicentre, single-arm, phase 2b study. Lancet Oncol 24:772-782, 2023 [DOI] [PubMed] [Google Scholar]
- 35.Kulukian A, Lee P, Taylor J, et al. : Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther 19:976-987, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Peterson S, de Vries P, Piasecki J, et al. : Tucatinib, a HER2 selective kinase inhibitor, is active in patient derived xenograft (PDX) models of HER2-amplified colorectal, esophageal and gastric cancers. Ann Oncol 28:576, 2017. 27993806 [Google Scholar]
- 37.Curigliano G, Mueller V, Borges V, et al. : Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): Final overall survival analysis. Ann Oncol 33:321-329, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Lin NU, Borges V, Anders C, et al. : Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 38:2610-2619, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin NU, Murthy RK, Abramson V, et al. : Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: Updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol 9:197-205, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartley AN, Washington MK, Colasacco C, et al. : HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 35:446-464, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Bekaii-Saab TS, Bridgewater J, Normanno N: Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol 32:1111-1126, 2021 [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarti S, Kamgar M, Mahipal A: Targeted therapies in advanced biliary tract cancer: An evolving paradigm. Cancers (Basel) 12:2039, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciombor KK, Goff LW: Current therapy and future directions in biliary tract malignancies. Curr Treat Options Oncol 14:337-349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valle JW, Borbath I, Khan SA, et al. : Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v28-v37, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Hollebecque A, Tselikas L, Ducreux MP, et al. : Genomic landscape and efficacy of precision medicine in biliary tract cancers. Ann Oncol 31:S267-S268, 2020 [Google Scholar]
- 46.Okamura R, Kurzrock R, Mallory RJ, et al. : Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int J Cancer 148:702-712, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang G, Liu Q, Wu Y, et al. : The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 127:2238-2250, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.00606.








