Abstract
This study aimed to determine the regulatory mechanism of dietary zinc lactate (ZL) supplementation on intestinal oxidative stress damage in a paraquat (PQ)-induced piglet model. Twenty-eight piglets (mean body weight 9.51 ± 0.23 kg) weaned at 28 d of age were randomly divided into control, ZL, PQ, and ZL + PQ groups (n = 7 in each group). The ZL-supplemented diet had little effect on growth performance under normal physiological conditions. However, under PQ challenge, ZL supplementation significantly improved average daily gain (P < 0.05) and reduced the frequency of diarrhea. ZL improved intestinal morphology and ultrastructure by significantly increasing the expression level of the jejunal tight junction protein, zonula occludens-1 (ZO-1) (P < 0.05), and intestinal zinc transport and absorption in PQ-induced piglets, which reduced intestinal permeability. ZL supplementation also enhanced the expression of antioxidant and anti-inflammatory factor-related genes and decreased inflammatory cytokine expression and secretion in PQ-induced piglets. Furthermore, ZL treatment significantly inhibited the activation of constitutive androstane receptor (CAR) signaling (P < 0.01) in PQ-induced piglets and altered the structure of the gut microbiota, especially by significantly increasing the abundance of beneficial gut microbes, including UCG_002, Ruminococcus, Rikenellaceae_RC9_gut_group, Christensenellaceae_R_7_group, Treponema, unclassified_Christensenellaceae, and unclassified_Erysipelotrichaceae (P < 0.05). These data reveal that pre-administration of ZL to piglets can suppress intestinal oxidative stress by improving antioxidant and anti-inflammatory capacity and regulating the crosstalk between CAR signaling and gut microbiota.
Keywords: Zinc lactate, Intestinal barrier, Oxidative stress, Inflammation, Constitutive androstane receptor, Gut microbiota
1. Introduction
Oxidative stress increases intestinal permeability, which is detrimental to maintaining the function and integrity of the intestinal epithelial barrier and disrupts the microbiota balance, thus causing diarrhea or reducing growth performance (Gresse et al., 2017; Moeser et al., 2007). Minerals are essential trace substances for normal physiological functions in animals and are crucial for the balance of the gut microbiota and intestinal function under normal physiological and stress states (Skalny et al., 2021). For example, zinc (Zn), is an essential trace element and an activator or a component of various enzymes in mammals, plays an important role in promoting animal growth and reproduction, and may be critical for maintaining the integrity of morphology and function of the intestinal mucosa (Andreini et al., 2006; Powell, 2000; Vallee and Falchuk, 1993). Under normal conditions, adequate Zn in the diet could alleviate oxidative stress damage, bacterial infection, and diarrhea by increasing intestinal tight junction protein expression, decreasing intestinal permeability, reducing intestinal villous cell apoptosis, and modulating the Th1 immune response (Crane et al., 2007; Sturniolo et al., 2001; Zhang and Guo, 2009). However, Zn deficiency in animals could disrupt intestinal barrier function and multiple systems including the digestive, neurological, immune, and endocrine systems, leading to intestinal dysfunction, T-lymphocyte reduction, oxidative stress, and inflammatory cell infiltration. These disruptions could, in turn, retard growth and cause diseases (Overbeck et al., 2008; Shankar and Prasad, 1998; Shi et al., 1998). Hence, an optimal dose of Zn used as a dietary supplement is important for helping maintain the homeostasis of gut microbiota and intestinal function. In contrast, dietary Zn concentrations that are too high or too low result in ecological dysbiosis of the gut microbiota (Reed et al., 2015).
Zinc oxide (ZnO), zinc sulfate (ZnSO4), basic zinc chloride, and organic forms of Zn that include zinc-amino acid complexes, zinc proteinate, and zinc lactate (ZL) are the main types of Zn that can be used in feedstuffs in China. The bioavailability of organic Zn is higher than that of inorganic Zn, which can decrease the amount of Zn addition, reduce heavy metal residues, and minimize environmental pollution by heavy metals (Diao et al., 2021; Long et al., 2022).
ZL is an organic acid-trace element chelate that has high solubility and dialysis. ZL readily binds ligands or metal carriers in the intestine and is delivered to cells. Hence, it has relatively high bioavailability (Meng et al., 2021). ZL improves growth performance and intestinal barrier function of weaned piglets, increases the proportion of beneficial bacteria, such as Lactobacilli, and reduces the proportion of pathogenic Escherichia coli (Diao et al., 2021). In vitro experiments also showed that ZL can promote the proliferation of IPEC-J2 intestinal porcine epithelial cells and upregulate the expression of Zn transporter-related genes. Moreover, ZL can reduce hydrogen peroxide-induced apoptosis and production of reactive oxide species (ROS) (Tang et al., 2020). Recent studies reported that the constitutive androstane receptor (CAR) nuclear receptor is mainly distributed in the liver and intestine and is closely related with cell proliferation and apoptosis, mineral element transport, hormone secretion, and energy metabolism in animals (Wang et al., 2012; Xiang et al., 2023; Yan et al., 2015). However, the effect of ZL supplementation on gut microbiota and CAR signaling pathway in oxidative stress piglets is unclear. The present study investigated whether ZL supplementation could alleviate intestinal oxidative stress by regulating CAR activation and supporting the gut microbiota balance in weaned piglets.
2. Materials and methods
2.1. Animal ethics statement
The handling of all experimental animals was approved by the Animal Welfare Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (20220056; Changsha, China) and all animal experiments complied with the ARRIVE guidelines.
2.2. Animal and experimental design
A total of 28 healthy Duroc × (Landrace × Yorkshire) piglets with similar body weight (BW = 9.51 ± 0.23 kg) were weaned at 28 d of age. The basal diet (Supplementary Table S1) meets the nutritional requirements of pigs according to the recommendations of the National Research Council (National Research Council, 2012). The whole experiment was divided into two phases. In phase 1, piglets in the control (CON) and ZL groups (n = 14 per group) were routinely fed for 4 weeks. In phase 2, each group of piglets was divided randomly into two sub-groups: paraquat (PQ) and ZL + PQ (n = 7 per treatment group). Piglets were fed a basal diet containing 80 mg/kg ZnSO4 (CON and PQ groups) or 80 mg/kg ZL (ZL and ZL + PQ groups). Piglets in the PQ and ZL + PQ groups were intraperitoneally injected with PQ (Chengdu HuaXia Chemical Reagent, Chengdu, China) at a dose of 8 mg/kg BW on d 28, 30, and 32. The CON and ZL groups were injected with the same volume of saline until slaughter on d 33.
2.3. Chemical analyses
Crude protein was detected by the national standard of China (GB/T 6432- 2018) using a flow injector (AA3, Seal, Norderstedt, Germany). Ether extracts were detected via national standard (GB/T 6433-2006) using a Soxhlet extractor (Soxtherm 6, Gerhardt, Nordrhein-Westfalen, Germany). Crude fiber was determined by the national standard of China (GB/T 6434-2022) using an automatic fiber analyzer (Fibretherm FT12, Gerhardt, Nordrhein-Westfalen, Germany). Calcium and phosphorus were determined by standards (GB/T 6436-2018 and GB/T 6437-2018) using an inductively coupled plasma emission spectrometer (5110 ICP-OES, Agilent, California, USA). Amino acids were determined by the national standard of China (GB/T 18246-2019) using an amino acid analyzer (L8900, Hitachi, Tokyo, Japan).
2.4. Sample collection
Feed intake and diarrhea were recorded daily for each group of piglets, and BW was recorded weekly. Average daily feed intake (ADFI), average daily gain (ADG), feed conversion ratio (FCR), and diarrhea rate were calculated from the records. At the end of the experiment, all piglets were anesthetized with sodium pentobarbital (60 mg/kg BW), followed by carotid artery bleeding to cause death. Blood samples were collected from the anterior vena cava and subsequently centrifuged at 3,000 × g for 10 min at 4 °C. The collected jejunum, ileum, and colonic chyme were stored at −80 °C.
2.5. Serum physiological and biochemical properties
The serum levels of malondialdehyde (MDA), total antioxidant capacity (T-AOC), and superoxide dismutase (SOD) were measured using an Infinite M200 PRO microplate reader (Tecan, Männedorf, Switzerland) with kits produced by Beijing Boxbio Science & Technology Co., Ltd as previously reported (Tang et al., 2020). Serum glutathione peroxidase (GSH-Px), glutathione (GSH), oxidized glutathione (GSSG), diamine oxidase (DAO), intestinal fatty acid binding protein (iFABP), interleukin-1 beta (IL-1β), IL-10, IL-12, and interferon-gamma (IFN-γ) levels were measured using ELISA kits (Jiangsu Meimian Industrial, Jiangsu, China) and the aforementioned Infinite M200 PRO microplate reader.
2.6. Intestinal histomorphology
The jejunum and ileum were fixed in 4% formaldehyde. After dehydration, paraffin embedding, sectioning, and hematoxylin and eosin staining, intestinal histomorphology was observed by fluorescent microscopy using a model BX51 microscope (Olympus, Tokyo, Japan) as previously described (He et al., 2022b). The ileum was fixed with 3% glutaraldehyde, semithin sections were stained with methylene blue, ultrathin sections were stained with uranyl acetate and lead citrate, and microvilli were observed by transmission electron microscopy (TEM) using a JEM-1400-FLASH microscope (JEOL, Tokyo, Japan) as described previously (Hu et al., 2022).
2.7. Cell apoptosis
Ileal epithelial apoptosis was determined using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis assay kit (Beyotime Biotech, Shanghai, China). Fluorescence signals were observed using a model DM3000 fluorescence microscope (Leica, Shanghai, China) as previously described (Xiang et al., 2023).
2.8. Immunohistochemical analysis
The location of Claudin 1, Occludin, and ZO-1 proteins in the jejunum and ileum was determined using immunohistochemical analysis as previously described (He et al., 2017). Sections were incubated with antibodies against the three proteins (all from Proteintech, Rosemont, IL, USA). Protein expression levels were expressed as the average optical density using the aforementioned BX51 microscope at 400× magnification.
2.9. Real-time PCR analysis
Total RNA was extracted from the jejunum and ileum using TRIzol (Beyotime, Shanghai, China). cDNA was synthesized using an Evo M-MLV reverse transcription kit (Accurate, Changsha, China). Each sample was evaluated three times using the SYBR Green Premix Pro Taq HS qPCR Kit (Accurate) as previously described (He et al., 2022a). The primers used are shown in Supplementary Table S2.
2.10. Western blot analysis
The jejunum was treated with cytosolic and nuclear extraction reagents to extract nuclear proteins. These proteins were used to determine the levels of CAR (Abcam, Cambridge, MA, USA), retinoid X receptor α (RXRα) (Proteintech, Wuhan, China), and PCNA (Proteintech, Wuhan, China). The procedural details have been described previously (He et al., 2018).
2.11. Gut microbiota profile
After extraction of genomic DNA from colonic chyme, PCR amplification was performed and the products were quantified and homogenized. A sequencing library was created, followed by double-end sequencing using a Novaseq 6000 device (Illumina, San Diego, CA, USA). Quality filtering, data splicing, and denoising were performed on the sequencing results to obtain the final valid data. Sequencing services were performed by Beijing Biomarker Technologies Co., Ltd (BMKcloud, Beijing, China). Alpha and Beta diversity were visualized using QIIME2 execution and R software (v3.2.0), and correlation heat maps between differential microbes and antioxidant, anti-inflammatory, and zinc transporter carriers were constructed using Spearman correlation analysis.
2.12. Statistical analysis
Data were analyzed by a t-test or two-way ANOVA using SPSS 20.0 software (IBM-SPSS Inc., Chicago, IL, USA). The statistical model included the effects of challenge (saline or PQ), diet (ZnSO4 or ZL), and their interactions. P < 0.05 indicates a significant difference, and Duncan's method was used for multiple comparisons to evaluate the differences among the treatments. Results are expressed as the mean ± standard error of the mean.
3. Results
3.1. Growth performance and intestinal morphology in weaned piglets
Under normal conditions, dietary ZL supplementation showed a significant increase in FCR at 14 to 21 d (P = 0.017), but no significant effect on growth performance at other stages (Table 1). In the first two weeks, there was no significant difference in the diarrhea rate of piglets fed the basal and ZL diets. The diarrhea rate was higher for piglets in the ZL group. However, these piglets had diarrhea before the experiment began. In the next two weeks, compared with that of the CON group, the ZL treatment had the greatest reduction in the diarrhea rate. After PQ challenge, the ADG of the PQ group was significantly decreased (P < 0.05) and the value was negative. However, compared with that of the PQ group, the ZL + PQ group had significantly increased ADG (P < 0.05) and the diarrhea rate was decreased by 49.96% (Fig. 1A–E).
Table 1.
Effect of zinc lactate (ZL) on growth performance of weaned piglets.
| Item | CON | ZL | P-value |
|---|---|---|---|
| 1 to 7 d | |||
| IBW, kg | 9.47 ± 0.36 | 9.54 ± 0.30 | 0.877 |
| FBW, kg | 11.98 ± 0.58 | 12.25 ± 0.46 | 0.722 |
| ADG, kg/d | 0.37 ± 0.03 | 0.38 ± 0.02 | 0.937 |
| ADFI, kg/d | 0.55 ± 0.03 | 0.55 ± 0.03 | 0.887 |
| FCR, % | 1.55 ± 0.09 | 1.47 ± 0.07 | 0.505 |
| DR, % | 9.52 ± 2.83 | 19.39 ± 4.04 | 0.069 |
| 7 to 14 d | |||
| IBW, kg | 11.98 ± 0.58 | 12.25 ± 0.46 | 0.722 |
| FBW, kg | 14.78 ± 0.64 | 14.61 ± 0.61 | 0.851 |
| ADG, kg/d | 0.40 ± 0.03 | 0.34 ± 0.03 | 0.145 |
| ADFI, kg/d | 0.74 ± 0.07 | 0.62 ± 0.05 | 0.173 |
| FCR, % | 1.89 ± 0.17 | 1.88 ± 0.10 | 0.956 |
| DR, % | 4.76 ± 1.68 | 7.14 ± 2.20 | 0.407 |
| 14 to 21 d | |||
| IBW, kg | 14.78 ± 0.64 | 14.61 ± 0.61 | 0.851 |
| FBW, kg | 17.95 ± 0.70 | 17.18 ± 0.80 | 0.498 |
| ADG, kg/d | 0.53 ± 0.03 | 0.43 ± 0.04 | 0.075 |
| ADFI, kg/d | 0.97 ± 0.05 | 0.93 ± 0.07 | 0.718 |
| FCR, % | 1.84 ± 0.09 | 2.30 ± 0.15 | 0.017 |
| DR, % | 1.19 ± 1.19 | 1.02 ± 1.02 | 0.915 |
| 21 to 28 d | |||
| IBW, kg | 17.95 ± 0.70 | 17.18 ± 0.80 | 0.498 |
| FBW, kg | 21.62 ± 0.83 | 20.57 ± 0.97 | 0.441 |
| ADG, kg/d | 0.61 ± 0.06 | 0.56 ± 0.03 | 0.427 |
| ADFI, kg/d | 1.05 ± 0.06 | 0.99 ± 0.05 | 0.370 |
| FCR, % | 1.79 ± 0.12 | 1.82 ± 0.15 | 0.882 |
| DR, % | 0.00 | 0.00 | |
ZL = zinc lactate; IBW = initial body weight; FBW = final body weight; ADG = average daily gain; ADFI = average daily feed intake; FCR = feed conversion ratio; DR = diarrhea rate. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
Fig. 1.
Effect of zinc lactate (ZL) on growth performance and intestinal morphology in weaned piglets. (A–E) The initial body weight (IBW), final body weight (FBW), average daily feed intake (ADFI), average daily gain (ADG), and diarrhea rate (DR) for different groups after paraquat (PQ) challenged. (F) Representative jejunal and ileal morphology of the Hematoxylin and Eosin (H&E) staining results (magnification, 100×; scale bars = 100 μm). (G) Villus height of jejunum and ileum in weaned piglets. (H) Crypt depth of jejunum and ileum in weaned piglets. (I) Vellus height to crypt depth ratios of jejunum and ileum in weaned piglets. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
Compared with that in the CON group, ZL treatment markedly increased (P < 0.05) villus height in the jejunum of piglets (Fig. 1G). PQ exposure damaged jejunal and ileal morphology (Fig. 1F). Moreover, the villus height and villus height/crypt depth in the jejunum and ileum were markedly decreased (P < 0.05; Fig. 1G, I). In contrast, ZL treatment significantly increased (P < 0.05) villus height in the ileum (Fig. 1G). No PQ challenge × ZL diet interaction effect was observed in the jejunal and ileal morphology of piglets.
3.2. Intestinal barrier function in paraquat (PQ)-induced weaned piglets
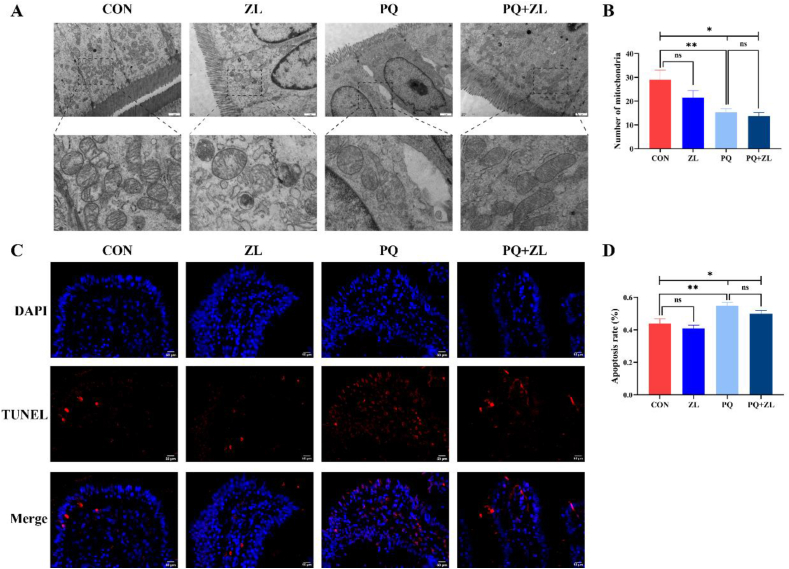
Under normal conditions, the intestinal ultrastructure of the ZL and CON groups showed no noticeable differences (Fig. 2A). Under PQ stimulation, the ileal microvilli were short and thick, loosely arranged, and morphologically irregular. Tight junctions were not obvious and cell gaps were widened, and mitochondrial morphology was heterogeneous. Furthermore, the number of mitochondria was significantly reduced (P < 0.01; Fig. 2B), with reduced and broken cristae evident. In PQ-induced piglets treated with ZL, the microvilli were tightly arranged without breakage, tight junctions were dense and undamaged, and mitochondrial morphology was normal, and the number of mitochondria was not significantly changed (Fig. 2B). TUNEL staining showed that PQ treatment led to massive apoptosis of ileal epithelial cells in weaned piglets compared to that in the CON group (P < 0.01; Fig. 2C–D). In contrast, ZL administration reduced the apoptosis rate by 9.10% (Fig. 2D).
Fig. 2.
Effect of zinc lactate (ZL) on ileal ultrastructure and apoptosis rate in weaned piglets. (A) Epithelial cells ultrastructure in the ileum (magnification, 15,000×; scale bars = 1 μm). (B) The number of mitochondria in ileal epithelial cells. (C) Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of the ileum (magnification, 400×; scale bars = 50 μm). (D) Quantitation of apoptosis rate in the ileum. PQ = paraquat. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
To investigate the effect of ZL on intestinal barrier function, we detected the localization and expression levels of the Occludin, Claudin-1, and ZO-1 tight junction proteins and intestinal permeability markers (DAO and iFABP). Compared to the CON group, ZL administration had no adverse effect on tight junction proteins or intestinal permeability (Fig. 3A–F). However, in piglets injected with PQ, significantly inhibited expression was evident for jejunal Occludin (P < 0.05) and ZO-1 (P < 0.01), and ileal Claudin-1 (P < 0.01), Occludin (P < 0.01), and ZO-1 (P < 0.01). ZL administration significantly increased (P < 0.05) expression of jejunal ZO-1 in PQ-induced piglets. After PQ challenge, intestinal permeability was significantly altered. iFABP activity in the PQ group was markedly higher (P < 0.01) than that in the CON group, and the DAO content showed an increasing trend (P = 0.053; Fig. 3E–F). However, ZL treatment did not significantly improve this undesirable damage.
Fig. 3.
Effect of zinc lactate (ZL) on intestinal tight junction protein and permeability in weaned piglets. (A–B) Immunohistochemical staining of Claudin-1, Occludin, and ZO-1 in the jejunum and ileum (magnification, 400×; scale bars = 50 μm). (C–D) The relative protein expression of Claudin-1, Occludin, and ZO-1 in the jejunum and ileum, expressed as average optical density (AOD). (E–F) Serum intestinal fatty acid binding protein (iFABP) and diamine oxidase (DAO) levels. PQ = paraquat. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
3.3. Antioxidant capacity and inflammatory response in paraquat (PQ)-induced weaned piglets
Compared to that in the CON group, ZL supplementation significantly increased serum levels of T-AOC (P < 0.05; Fig. 4C), GSH (P < 0.05; Fig. 4E), GSSG (P < 0.05; Fig. 4E), jejunal GSH (P < 0.01; Fig. 4F), and GSSG (P < 0.01; Fig. 4G). ZL supplementation also significantly upregulated the mRNA expression of jejunal MnSOD (P < 0.05; Fig. 4M). PQ treatment resulted in significant increases in serum MDA (P < 0.01; Fig. 4A), GSH-PX (P < 0.01; Fig. 4B), and GSSG (P < 0.05) levels but markedly decreased serum T-AOC (P < 0.05), SOD (P < 0.01) (Fig. 4D), serum and jejunal GSH (P < 0.01 and P < 0.05, respectively), and serum, jejunal, and ileal GSH/GSSG levels (P < 0.01, P < 0.05, and P < 0.01, respectively) (Fig. 4H). The mRNA expression of jejunal GPX4 was significantly upregulated (P < 0.01) in the PQ group (Fig. 4M). In contrast, ZL supplementation dramatically reduced (P < 0.05) serum MDA and GSSG levels and enhanced serum SOD (P < 0.05), jejunal GSH (P < 0.01), and serum and jejunal GSH/GSSG levels (P < 0.05 and P < 0.01, respectively). However, ZL supplementation had no significant effect on the mRNA expression of jejunal GPX4, MnSOD, GCLC, or GCLM.
Fig. 4.
Effects of zinc lactate (ZL) on antioxidant capacity and inflammatory response in weaned piglets. (A–D) The malondialdehyde (MDA), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and superoxide dismutase (SOD) levels in the serum. (E) The glutathione (GSH) and oxidized glutathione (GSSG) levels in the serum. (F–G) The GSH and GSSG levels in the jejunum and ileum. (H) The GSH/GSSG levels in the serum, jejunum and ileum. (I–L) The interleukin-1β (IL-1β), interferon-γ (IFN-γ), interleukin-10 (IL-10), and interleukin-12 (IL-12) levels in the serum. (M) The mRNA expression levels of glutathione peroxidase 4 (GPX4), manganese superoxide dismutase (MnSOD), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) in the jejunum. (N) The mRNA expression levels of IL-1β, IFN-γ, IL-10, and IL-12 in the jejunum. PQ = paraquat. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
The inflammatory response is typically accompanied by oxidative stress. Compared to that in the CON group, the ZL group displayed significantly reduced serum IL-1β levels (P < 0.05; Fig. 4I). PQ challenge resulted in the significant elevation in serum levels of IL-1β (P < 0.01) and IFN-γ (P < 0.05) (Fig. 4J) and significantly increased mRNA expression of jejunal IFN-γ (P < 0.05; Fig. 4N). Notably, ZL supplementation decreased serum IL-1β (P < 0.05) levels in PQ-induced piglets, downregulated (P < 0.01) jejunal IFN-γ mRNA levels, and upregulated (P < 0.01) mRNA expression level IL-10 (Fig. 4N).
3.4. Gene and protein expression of Zn transporters and CAR pathway-related targets in paraquat (PQ)-induced weaned piglets
Compared to that in the CON group, ZL supplementation significantly upregulated the mRNA expression of ZnT-1 (P < 0.01; Fig. 5B). After PQ challenge, ZL supplementation markedly upregulated the mRNA expression levels of Zip4 (P < 0.05; Fig. 5A), ZnT-1 (P < 0.01), CRIP1 (P < 0.01) (Fig. 5D), and CRIP2 (P < 0.01; Fig. 5E).
Fig. 5.
Effects of zinc lactate (ZL) on zinc transporters and constitutive androstane receptor (CAR)-regulated pathway genes in weaned piglets. (A–E) The mRNA expression levels of Zrt/Irt-like protein 4 (Zip4), zinc transporter-1 (ZnT-1), metallothionein 1A (MT1A), cysteine-rich intestinal protein-1 (CRIP1), and cysteine-rich intestinal protein-2 (CRIP2) in the jejunum. (F–J) The mRNA expression levels of CAR, retinoid X receptor α (RXRα), heat shock protein 90 (HSP90), cytoplasmic CAR retention protein (CCRP), and protein phosphatase 2Ac (PP2Ac) in the jejunum. (K–O) The mRNA expression levels of cytochrome P450 1A2 (CYP1A2), cytochrome P450 2B22 (CYP2B22), cytochrome P450 3A29 (CYP3A29), glutathione S-transferase Alpha 1 (GSTA1), and glutathione S-transferase Alpha 2 (GSTA2) in the jejunum. (P) The nuclear protein expression levels of CAR and RXRα in the jejunum. PQ = paraquat. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
Subsequently, we examined the effects of ZL on the CAR pathway in the jejunum. Compared to that in the CON group, no effect of ZL supplementation on the expression of CAR signaling-related genes and proteins was observed. The ZL supplementation did significantly reduce the mRNA expression of HSP90 (P < 0.05; Fig. 5H). However, PQ dramatically upregulated the mRNA expression of CAR (P < 0.05), RXRα (P < 0.01), and PP2Ac (P < 0.01) (Fig. 5F–G, J), significantly upregulated the mRNA expression of the target gene CYP1A2 (P < 0.05; Fig. 5K), and significantly downregulated the mRNA expression of GSTA1 (P < 0.05; Fig. 5N). Moreover, the PQ challenge significantly increased the expression of CAR and RXRα nuclear proteins (both P < 0.01; Fig. 5P). In contrast, ZL supplementation significantly downregulated the mRNA expression of CAR, RXRα, HSP90, PP2Ac (all P < 0.01), and CYP2B22 (P < 0.05), while significantly decreasing the protein expression of CAR and RXRα (both P < 0.01).
3.5. Gut microbiota profile in paraquat (PQ)-induced weaned piglets
We performed 16S rRNA sequencing to evaluate the changes in the gut microbiota during dietary ZL supplementation. Alpha diversity measured using the Chao1 (Fig. 6A) and Shannon indices (Fig. 6B) revealed no difference between the ZL and CON groups. However, the Chao1 and Shannon indices were significantly lower (P < 0.01) in the PQ group, and ZL treatment improved the reduction of alpha diversity in PQ-induced piglets. Partial least squares-discriminant analysis separated the overall microbiota composition into three clusters: CON, PQ, and ZL and ZL + PQ (Fig. 6C). Further explorations of the distribution of the gut microbiota revealed that, at the phylum level, PQ treatment decreased the relative abundance of Bacteroidota but increased the relative abundance of Firmicutes and Actinobacteriota (Fig. 6D). At the genus level, Family_XIII_UCG_001, Mogibacterium, and Dialister were the main microbes (Supplementary Fig. S1). However, the result of linear discriminant analysis (LDA) effect size (LDA score > 3.0) showed that the prominent gut microbes in the ZL group were opposite to those in the PQ group (Fig. 6F). At the phylum level, the relative abundance of Bacteroidota in the ZL group was higher than that in the PQ group, and the relative abundance of Actinobacteriota in the ZL group was lower than that in the PQ group (Fig. 6D). At the genus level, ZL treatment mainly increased (P < 0.05, unless noted) the relative abundance of UCG_002, Christensenellaceae_R_7_group (P < 0.01), Ruminococcus, Rikenellaceae_RC9_gut_group, unclassified_Christensenellaceae, Treponema (P < 0.01), and unclassified_Erysipelotrichaceae and reduced (P < 0.05) the relative abundance of Olsenella (Fig. 6G) in PQ-induced piglets. Finally, the differential gut microbe profile correlated with oxidative stress, inflammatory cytokines, and Zn transporters (Fig. 6H). Olsenella was significantly positively correlated (P < 0.05) with MDA and GSSG and negatively correlated (P < 0.01) with T-AOC. In addition, UCG_002, Treponema, Rikenellaceae_RC9_gut_group, Ruminococcus, Christensenellaceae_R_7_group, unclassified_Christensenellaceae, and unclassified_Erysipelotrichaceae were positively correlated (P < 0.05) with antioxidants and Zn transporters and negatively correlated with (P < 0.05) MDA, GSSG, and IL-1β.
Fig. 6.
Effects of zinc lactate (ZL) on gut microbiota in weaned piglets. (A–B) Chao1 and Shannon index of operational taxonomic unit (OTU) levels. (C) Partial least-squares discriminant analysis (PLS-DA) score plot of the four groups. (D–E) Relative abundance of bacteria classified at phylum-level and genus-level taxonomy. (F) Linear discriminant analysis Effect Size (LEfSe) test showing the distinctive gut microbes (LDA > 3.0) in the four groups. (G) Differences in microbes between the PQ group and ZL + PQ group. (H) Correlation between gut microbiota and antioxidant, anti-inflammatory parameters and zinc transporters at the genus levels. PQ = paraquat. ∗, P < 0.05; ∗∗, P < 0.01; ns, P > 0.05. n = 7.
4. Discussion
Zn regulates the appetite of animals and contributes to increased feeding and growth (Shay and Mangian, 2000). Diao et al. (2021) found that organic Zn compounds significantly reduced the feed-to-gain value and diarrhea rate when the diet of weaned piglets was supplemented with both inorganic zinc (ZnSO4) and organic zinc (Gly-Zn and ZL) (Diao et al., 2021). However, other studies have shown that the zinc-amino acid complex does not significantly improve the growth performance of piglets compared to the same dose of ZnO in the diets (Case and Carlson, 2002). In our study, dietary ZL supplementation had little growth-promoting effects under normal conditions but significantly increased ADG and reduced the diarrhea rate under oxidative stress conditions. It is possible that piglets appear less sensitive to different Zn sources when their nutritional needs are met, but ZL supplementation may enhance the bioavailability of Zn and provide more energy for piglet growth and antioxidative stress.
Zn transporters control Zn uptake and excretion through organelle membranes and are essential for regulating Zn distribution and maintaining Zn homeostasis (Ohashi and Fukada, 2019). Regulation of Zn homeostasis occurs primarily in the gastrointestinal tract, where this homeostasis contributes to the maintenance of intestinal barrier function and promotes the repair and regeneration of the intestinal mucosa (Ohashi and Fukada, 2019). Zn-regulated transporters and iron-regulated transporter-like protein (Zip) and cysteine-rich intestinal protein (CRIP) are involved in the transport and absorption of Zn ions in the small intestine, whereas Zn transporter (ZnT) and metallothionein (MT) is mainly involved in the excretion of Zn into the gastrointestinal tract (Jeong and Eide, 2013; Liuzzi and Cousins, 2004; Levenson et al., 1993). Other authors have reported that chitosan-chelated Zn treatment upregulated the gene and protein expression of ZnT1, Zip4, and Zip5 in piglets to regulate Zn homeostasis (Lv et al., 2016). Furthermore, expressions of the Zn transporter Zip4 and divalent metal transporter are upregulated at low concentrations of Zn to promote Zn uptake, whereas the efflux transporters ZnT1 and MT1 are upregulated at high concentrations of Zn to enhance Zn efflux and maintain Zn homeostasis (Huang et al., 2016; Shen et al., 2008). In the present study, ZL administration upregulated the expression of Zn transporter-related genes (Zip4, ZnT-1, CRIP1, and CRIP2) under both normal and oxidative stress conditions. Although our results did not reveal whether lactic acid plays a role in this process, our previous results confirm that the combination of lactic acid and ZnSO4 could elevate MT1A levels and decrease CRIP1 levels in IPEC-J2 cells compared to ZnSO4 alone, which appeared to be detrimental to the maintenance of Zn homeostasis (Tang et al., 2020). These results suggest that the chelated form of lactic acid and ZnSO4 can promote Zn ion transport and absorption and maintain Zn homeostasis by increasing intestinal Zn excretion, improving intestinal barrier function.
Integrity of the intestinal barrier is critical for nutrient absorption and immune function. However, oxidative stress disrupts the intestinal structure, damages intestinal tight junction proteins, increases permeability, and causes epithelial cell apoptosis (Wang et al., 2020). The amino acid Zn complex in the diet alleviates heat stress-induced reduction in ileal villus height and improves intestinal integrity in growing pigs (Pearce et al., 2015). In the present study, dietary ZL supplementation increased jejunal and ileal villus heights in stressed piglets and improved microvillus structure, tight junctions, and mitochondrial morphology in ileal epithelial cells but did not reduce apoptosis. These changes may be related to the dose of Zn added, given the prior descriptions that supplementation with 50 mg/kg chitosan Zn chelate had no differential effect on apoptosis compared to 100 mg/kg ZnSO4, which significantly reduced ileal apoptosis and increased the ratio of villi height to crypt depth in the small intestine (Han et al., 2014). Claudin-1, Occludin, and ZO-1 proteins are the most critical parts of the tight junction structure and play primary roles in maintaining intestinal permeability (Tsukita et al., 2001). High dietary Zn can significantly upregulate mRNA and protein expression of Occludin and ZO-1 in piglet ileal mucosa (Zhang and Guo, 2009). We observed that dietary ZL supplementation significantly upregulated the protein expression of ZO-1 in the jejunum of PQ-induced piglets and reduced intestinal permeability, which may contribute to the reduction of diarrhea in piglets experiencing oxidative stress.
Inadequate Zn supplementation increases production of ROS, heightens the vulnerability to oxidative stress, and causes inflammatory responses (Higashimura et al., 2020; Oteiza et al., 1995). In another study, the combination of chromium methionine complexes and Zn-amino acid complexes increased T-AOC and SOD activity and decreased serum MDA concentrations compared to ZnSO4 (Xu et al., 2017). We previously reported that ZL treatment increased GSH-PX and SOD activity in IPEC-J2 cells more than combination treatment with ZnSO4 and lactic acid and that lactate dehydrogenase and MDA levels increased after treatment with ZnSO4 and lactic acid (Tang et al., 2020). These findings indicate that ZL administration improves the antioxidant capacity of piglets by regulating antioxidant-related gene expression and antioxidant enzyme activity and reducing MDA levels; lactate plays a minimal role in these processes. Zn modulates intestinal immune function and has anti-inflammatory effects (Tapiero and Tew, 2003). Our results showed that dietary ZL supplementation reduced serum IL-1β levels and jejunal IFN-γ mRNA abundance and upregulated the mRNA expression of IL-10 in stressed piglets. These findings are consistent with the previous report that ZL treatment can downregulate the mRNA expression level of IL-12 and upregulate mRNA expression of IL-10 in the small intestine of grass carp (Song et al., 2017). Furthermore, Zn supplementation can increase T cell numbers and reduce cytokine secretion to improve immunity in piglets (Kloubert et al., 2018).
CAR signaling can regulate the expression of detoxification enzymes and antioxidant genes and induce the expression of IL-10, a key anti-inflammatory factor, to exert detoxification and antioxidant effects (Chen et al., 2021). Our results are similar to those of Du et al. (2017), who observed that PQ-induced oxidative stress phosphorylated CAR, prevented the formation of the CAR-CCRP-HSP90 complex, and promoted CAR nuclear translocation, which bound to RXRα, thereby regulating the expression of downstream target genes CYP450 and accelerating CAR activation. However, supplementation with ZL inhibited CAR nuclear translocation and the expression of CYP450 enzyme systems under oxidative stress conditions to maintain intestinal redox homeostasis. This may be because ZL administration provides more Zn to combine with these key enzymes, alleviating the response to oxidative stress in the body.
The host immune system and microbiota are closely linked. Dysregulation of the gut microbiota may affect host metabolism and health, causing chronic inflammation and metabolic disorders (Yan et al., 2011). Zn is an essential mineral for many bacteria and is involved in intestinal microbial barrier function against the invasion of pathogenic bacteria (Davis et al., 2009; Skalny et al., 2021). Zn deficiency results in decreased intestinal microbial diversity and growth of bacteria suitable for Zn concentration. These changes alter the intestinal microbial composition, leading to dysbiosis of the intestinal microecology (Reed et al., 2015). A previous study reported that Zn supplementation in broilers infected with Salmonella typhimurium improved intestinal barrier health by increasing gut microbiota diversity and reducing Salmonella populations (Shao et al., 2014). Our results showed that under normal conditions, ZL treatment had no effect on the diversity of the gut microbiota. However, under oxidative stress conditions, ZL administration significantly increased the diversity and composition of the gut microbiota, suggesting that ZL can improve the gut microbial balance of piglets under stressful conditions. In parallel with the increased diversity resulting from ZL treatment, we also observed some significant changes in the structure of the intestinal microbial community that may have a probiotic effect on the intestine of piglets. These changes included the enrichment of Rikenellaceae_RC9_gut_group, Treponema, Christensenellaceae_R_7_group, and unclassified_Erysipelotrichaceae, and the downregulation of Olsenella. Rikenellaceae_RC9_gut_group promotes lipid metabolism and enhances intestinal mucosal immune function to improve the health of the body (Fan et al., 2020; Zhou et al., 2018). Treponema is involved in the degradation of lignocellulose (Baniel et al., 2021). Furthermore, probiotic supplementation in stunted calves can increase the relative abundance of intestinal Treponema and improve growth (Du et al., 2018). The Christensenellaceae_R_7_group is involved in amino acid and lipid metabolism and exerts beneficial effects on organismal health by influencing host metabolism (Goodrich et al., 2014; Waters and Ley, 2019). Erysipelotrichaceae are associated with host lipid metabolism (Kaakoush, 2015). Some studies have reported an increase in the relative abundance of Erysipelotrichaceae in mice on a high-fat or western diet (Lohuis et al., 2019). However, we presently observed a significant reduction in body weight after PQ challenge, which may be related to the reduced relative abundance of Erysipelotrichaceae. Olsenella is highly correlated with inflammation, oxidative stress, and lack of intestinal integrity, and its imbalance causes dysregulation of the intestinal microecology (Wang et al., 2022). Subsequent correlation analyses suggested that these differential microbes upregulated by ZL administration were highly positively associated with antioxidant and Zn transport and significantly negatively correlated with oxidative stress and inflammation, whereas the opposite findings were observed for downregulated microbes. The findings indicate the improvement of intestinal health via the crosstalk between gut microbes and ZL metabolism.
5. Conclusion
The collective results of this study indicate that the ZL-supplemented diet had little effect on the growth performance of piglets under normal physiological conditions. However, ZL supplementation could reduce the rate of diarrhea and improve intestinal morphology in both normal and stressful conditions. In particular, dietary ZL supplementation could increase the ADG of piglets, improve intestinal barrier function and Zn transport and absorption, and enhance antioxidant capacity and immunity in response to PQ damage. We suggest that the mechanism underlying the protective effects of ZL against oxidative stress is its critical role in inhibiting CAR activation and altering gut microbiota diversity and structure. The efficacy of ZL suggests that it can be used to reduce oxidative stress and prevent diarrhea in animals and humans.
Author contributions
Wenjie Tang: Investigation, Conceptualization, Writing - original draft. Xuan Xiang: Visualization, Software, Data curation, Writing - original draft. Houfu Wang: Investigation, Software. Wentao Zhou: Investigation, Software. Liuqin He: Data curation, Writing - review & editing. Tiejun Li: Investigation, Writing - review & editing. Yulong Yin: Supervision, Writing - review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32172755, 32130099), Hunan Key Research and Development Plan (2022NK2023), Shandong Province Taishan Industry Leading Talents Project Blue Talents Project. Thanks to the Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.10.001.
Contributor Information
Liuqin He, Email: heliuqin@hunnu.edu.cn.
Tiejun Li, Email: tjli@isa.ac.cn.
Appendix supplementary data
The following is the Supplementary data to this article:
References
- Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- Baniel A., Amato K.R., Beehner J.C., Bergman T.J., Mercer A., Perlman R.F., Petrullo L., Reitsema L., Sams S., Lu A., Snyder-Mackler N. Seasonal shifts in the gut microbiome indicate plastic responses to diet in wild geladas. Microbiome. 2021;9:26. doi: 10.1186/s40168-020-00977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case C.L., Carlson M.S. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J Anim Sci. 2002;80:1917–1924. doi: 10.2527/2002.8071917x. [DOI] [PubMed] [Google Scholar]
- Chen M.L., Huang X., Wang H., Hegner C., Liu Y., Shang J., Eliason A., Diao H., Park H., Frey B., Wang G., Mosure S.A., Solt L.A., Kojetin D.J., Rodriguez-Palacios A., Schady D.A., Weaver C.T., Pipkin M.E., Moore D.D., Sundrud M.S. CAR directs T cell adaptation to bile acids in the small intestine. Nature. 2021;593:147–151. doi: 10.1038/s41586-021-03421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J.K., Naeher T.M., Shulgina I., Zhu C., Boedeker E.C. Effect of zinc in enteropathogenic Escherichia coli infection. Infect Immun. 2007;75:5974–5984. doi: 10.1128/IAI.00750-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L.M., Kakuda T., DiRita V.J. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol. 2009;191:1631–1640. doi: 10.1128/JB.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Yan J., Li S., Kuang S., Wei X., Zhou M., Zhang J., Huang C., He P., Tang W. Effects of dietary zinc sources on growth performance and gut health of weaned piglets. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.771617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.H., Xia J., Sun X.C., Li X.N., Zhang C., Zhao H.S., Zhu S.Y., Li J.L. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ Pollut. 2017;226:435–443. doi: 10.1016/j.envpol.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Du R., Jiao S., Dai Y., An J., Lv J., Yan X., Wang J., Han B. Probiotic Bacillus amyloliquefaciens C-1 improves growth performance, stimulates GH/IGF-1, and regulates the gut microbiota of growth-retarded beef calves. Front Microbiol. 2018;9:2006. doi: 10.3389/fmicb.2018.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P., Bian B., Teng L., Nelson C.D., Driver J., Elzo M.A., Jeong K.C. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020;14:302–317. doi: 10.1038/s41396-019-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., Spector T.D., Clark A.G., Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Han X.Y., Ma Y.F., Lv M.Y., Wu Z.P., Qian L.C. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br J Nutr. 2014;111:1405–1411. doi: 10.1017/S0007114513004042. [DOI] [PubMed] [Google Scholar]
- He L.Q., Zhou X.H., Huang N., Li H., Cui Z.J., Tian J.Q., Jiang Q., Liu S.J., Wu J., Li T.J., Yao K., Yin Y.L. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget. 2017;8:91965–91978. doi: 10.18632/oncotarget.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wu J., Tang W., Zhou X., Lin Q., Luo F., Yin Y., Li T. Prevention of oxidative stress by alpha-ketoglutarate via activation of CAR signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J Agric Food Chem. 2018;66:11273–11283. doi: 10.1021/acs.jafc.8b04470. [DOI] [PubMed] [Google Scholar]
- He L.Q., Zhou X.H., Liu Y.H., Zhou L.M., Li F.N. Fecal miR-142a-3p from dextran sulfate sodium-challenge recovered mice prevents colitis by promoting the growth of Lactobacillus reuteri. Mol Ther. 2022;30:388–399. doi: 10.1016/j.ymthe.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.Q., Zhou X.H., Wu Z.P., Feng Y.Z., Liu D., Li T.J., Yin Y.L. Glutamine in suppression of lipopolysaccharide-induced piglet intestinal inflammation: the crosstalk between AMPK activation and mitochondrial function. Anim Nutr. 2022;10:137–147. doi: 10.1016/j.aninu.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimura Y., Takagi T., Naito Y., Uchiyama K., Mizushima K., Tanaka M., Hamaguchi M., Itoh Y. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J Crohns Colitis. 2020;14:856–866. doi: 10.1093/ecco-jcc/jjz193. [DOI] [PubMed] [Google Scholar]
- Hu X., He X., Peng C., He Y., Wang C., Tang W., Chen H., Feng Y., Liu D., Li T., He L. Improvement of ulcerative colitis by aspartate via RIPK pathway modulation and gut microbiota composition in mice. Nutrients. 2022;14 doi: 10.3390/nu14183707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Zhuo Z., Fang S., Yue M., Feng J. Different zinc sources have diverse impacts on gene expression of zinc absorption related transporters in intestinal porcine epithelial cells. Biol Trace Elem Res. 2016;173:325–332. doi: 10.1007/s12011-016-0655-x. [DOI] [PubMed] [Google Scholar]
- Jeong J., Eide D.J. The SLC39 family of zinc transporters. Mol Aspects Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush N.O. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;5:84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloubert V., Blaabjerg K., Dalgaard T.S., Poulsen H.D., Rink L., Wessels I. Influence of zinc supplementation on immune parameters in weaned pigs. J Trace Elem Med Biol. 2018;49:231–240. doi: 10.1016/j.jtemb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Levenson C.W., Shay N.F., Leeambrose L.M., Cousins R.J. Regulation of cysteine-rich intestinal protein by dexamethasone in the neonatal rat. Proc Natl Acad Sci U S A. 1993;90:712–715. doi: 10.1073/pnas.90.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi J.P., Cousins R.J. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Lohuis M.A.M., Werkman C.C.N., Harmsen H.J.M., Tietge U.J.F., Verkade H.J. Absence of intestinal microbiota during gestation and lactation does not alter the metabolic response to a western-type diet in adulthood. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201800809. [DOI] [PubMed] [Google Scholar]
- Long L., Zhao X., Li H., Yan X., Zhang H. Effects of zinc lactate supplementation on growth performance, intestinal morphology, serum parameters, and hepatic metallothionein of Chinese yellow-feathered broilers. Biol Trace Elem Res. 2022;200:1835–1843. doi: 10.1007/s12011-021-02785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M., Fu X., Hu L., Yue X., Han X. The expression of zinc transporters changed in the intestine of weaned pigs exposed to zinc chitosan chelate. Biol Trace Elem Res. 2016;174:328–334. doi: 10.1007/s12011-016-0732-1. [DOI] [PubMed] [Google Scholar]
- Meng K., Chen L., Xia G., Shen X. Effects of zinc sulfate and zinc lactate on the properties of tilapia (Oreochromis Niloticus) skin collagen peptide chelate zinc. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2021.129043. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Ryan K.A., Nighot P.K., Blikslager A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- National Research Council . The National Academies Press; Washington, DC: 2012. Nutrient Requirements of Swine: Eleventh Revised Edition. [Google Scholar]
- Ohashi W., Fukada T. Contribution of zinc and zinc transporters in the pathogenesis of inflammatory bowel diseases. J Immunol Res. 2019;2019 doi: 10.1155/2019/8396878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza P.I., Olin K.L., Fraga C.G., Keen C.L. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823–829. doi: 10.1093/jn/125.4.823. [DOI] [PubMed] [Google Scholar]
- Overbeck S., Rink L., Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp. 2008;56:15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S.C., Fernandez M.V.S., Torrison J., Wilson M.E., Baumgard L.H., Gabler N.K. Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J Anim Sci. 2015;93:4702–4713. doi: 10.2527/jas.2015-9018. [DOI] [PubMed] [Google Scholar]
- Powell S.R. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- Reed S., Neuman H., Moscovich S., Glahn R.P., Koren O., Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Shao Y., Lei Z., Yuan J., Yang Y., Guo Y., Zhang B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar Typhimurium. J Microbiol. 2014;52:1002–1011. doi: 10.1007/s12275-014-4347-y. [DOI] [PubMed] [Google Scholar]
- Shay N.F., Mangian H.F. Neurobiology of zinc-influenced eating behavior. J Nutr. 2000;130:1493S–1499S. doi: 10.1093/jn/130.5.1493S. [DOI] [PubMed] [Google Scholar]
- Shen H., Qin H., Guo J. Cooperation of metallothionein and zinc transporters for regulating zinc homeostasis in human intestinal Caco-2 cells. Nutr Res. 2008;28:406–413. doi: 10.1016/j.nutres.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Shi H.N., Scott M.E., Stevenson M.M., Koski K.G. Energy restriction and zinc deficiency impair the functions of murine T cells and antigen-presenting cells during gastrointestinal nematode infection. J Nutr. 1998;128:20–27. doi: 10.1093/jn/128.1.20. [DOI] [PubMed] [Google Scholar]
- Skalny A.V., Aschner M., Lei X.G., Gritsenko V.A., Santamaria A., Alekseenko S.I., Prakash N.T., Chang J.S., Sizova E.A., Chao J.C.J., Aaseth J., Tinkov A.A. Gut microbiota as a mediator of essential and toxic effects of zinc in the intestines and other tissues. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222313074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.X., Jiang W.D., Liu Y., Wu P., Jiang J., Zhou X.Q., Kuang S.Y., Tang L., Tang W.N., Zhang Y.A., Feng L. Dietary zinc deficiency reduced growth performance, intestinal immune and physical barrier functions related to NF-κB, TOR, Nrf2, JNK and MLCK signaling pathway of young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;66:497–523. doi: 10.1016/j.fsi.2017.05.048. [DOI] [PubMed] [Google Scholar]
- Sturniolo G.C., Di Leo V., Ferronato A., D'Odorico A., D'Inca R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Tang W.J., Long J., Li T.J., Yang L.Y., Li J.Z., He L.Q., Li S.W., Kuang S.Y., Feng Y.Z., Chen H.S., Li F.L., Du Z.L., Yin Y.L. The associated regulatory mechanisms of zinc lactate in redox balance and mitochondrial function of intestinal porcine epithelial cells. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/8815383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Tew K.D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Wang Y.M., Ong S.S., Chai S.C., Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Zhang X., Lu Y., Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J Funct Foods. 2020;75 [Google Scholar]
- Wang Q., Zhan X., Wang B., Wang F., Zhou Y., Xu S., Li X., Tang L., Jin Q., Li W., Gong L., Fu A. Modified montmorillonite improved growth performance of broilers by modulating intestinal microbiota and enhancing intestinal barriers, anti-inflammatory response, and antioxidative capacity. Antioxidants. 2022;11:1799. doi: 10.3390/antiox11091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.L., Ley R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Wang H., Zhou W., Wang C., Guan P., Xu G., Zhao Q., He L., Yin Y., Li T. Glutathione protects against paraquat-induced oxidative stress by regulating intestinal barrier, antioxidant capacity, and CAR signaling pathway in weaned piglets. Nutrients. 2023;15:198. doi: 10.3390/nu15010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Liu L., Long S.-F., Piao X.-S., Ward T.L., Ji F. Effects of chromium methionine supplementation with different sources of zinc on growth performance, carcass traits, meat quality, serum metabolites, endocrine parameters, and the antioxidant status in growing-finishing pigs. Biol Trace Elem Res. 2017;179:70–78. doi: 10.1007/s12011-017-0935-0. [DOI] [PubMed] [Google Scholar]
- Yan A.W., Fouts D.E., Brandl J., Starkel P., Torralba M., Schott E., Tsukamoto H., Nelson K.E., Brenner D.A., Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Chen B., Lu J., Xie W. Deciphering the roles of the constitutive androstane receptor in energy metabolism. Acta Pharmacol Sin. 2015;36:62–70. doi: 10.1038/aps.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Zhou L., Xiao X., Zhang Q., Zheng J., Li M., Yu M., Wang X., Deng M., Zhai X., Li R. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front Endocrinol. 2018;9:516. doi: 10.3389/fendo.2018.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.