Abstract
Toxoplasma gondii is an obligate intracellular parasite in the phylum Apicomplexa that causes toxoplasmosis in humans and animals worldwide. Despite its prevalence, there is currently no effective vaccine or treatment for chronic infection. Although there are therapies against the acute stage, prolonged use is toxic and poorly tolerated. This study aims to explore the potential of repurposing topotecan and 10-hydroxycamptothecin (HCPT) as drugs producing double strand breaks (DSBs) in T. gondii. DSBs are mainly repaired by Homologous Recombination Repair (HRR) and Non-Homologous End Joining (NHEJ). Two T. gondii strains, RHΔHXGPRT and RHΔKU80, were used to compare the drug's effects on parasites. RHΔHXGPRT parasites may use both HRR and NHEJ pathways but RHΔKU80 lacks the KU80 protein needed for NHEJ, leaving only the HRR pathway. Here we demonstrate that topotecan and HCPT, both topoisomerase I venoms, affected parasite replication in a concentration-dependent manner. Moreover, variations in fluorescence intensity measurements for the H2A.X phosphorylation mark (γH2A.X), an indicator of DNA damage, were observed in intracellular parasites under drug treatment conditions. Interestingly, intracellular replicative parasites without drug treatment show a strong positive staining for γH2A.X, suggesting inherent DNA damage. Extracellular (non-replicating) parasites did not exhibit γH2A.X staining, indicating that the basal level of DNA damage is likely to be associated with replicative stress. A high rate of DNA replication stress possibly prompted the evolution of an efficient repair machinery in the parasite, making it an attractive target. Our findings show that topoisomerase 1 venoms are effective antiparasitics blocking T. gondii replication.
Keywords: Toxoplasma gondii, Topotecan, 10-Hydroxycamptothecin, DNA damage, Stress replication, DNA damage response
Graphical abstract
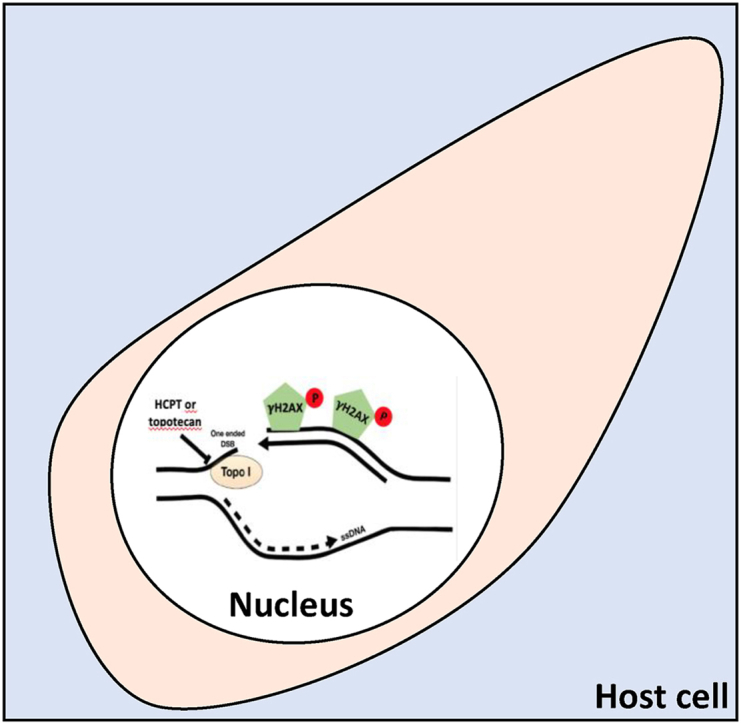
Highlights
-
•
Topotecan and 10-Hydroxycamptothecin (HCPT) block Toxoplasma gondii replication.
-
•
Topotecan and HCPT induce Double strand break (DSB) DNA damage.
-
•
High replicative tachyzoite presents a baseline DSB DNA damage efficiently repaired.
-
•
Combining these drugs with repair intervention shows promise as therapies.
1. Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite that belongs to the phylum Apicomplexa. It is responsible for toxoplasmosis, a world-wide distributed zoonotic infection that can be transmitted through ingestion of contaminated food or water, contact with infected cat feces, or congenitally from an infected mother to her fetus (Guo et al., 2015). It is estimated that one-third of humans have been exposed to the parasite and between five hundred and a billion humans are chronically infected (Tenter et al., 2000). Toxoplasmosis is a common opportunistic infection in HIV/AIDS patients, producing severe brain damage or even death (Basavaraju 2016). In immunocompetent patients, T. gondii is found in its latent form predominantly in nervous and muscle tissues. Recent studies have associated this chronic infection with dopamine and testosterone alterations that could interfere with human behavior and psychiatric disorders such as schizophrenia (Flegr 2007).
There is no vaccine against toxoplasmosis in humans and currently no treatment effective against the chronic stage. Current options for acute toxoplasmosis include sulfadiazine and pyrimethamine that are associated with dermatologic and bone marrow suppression secondary effects (Ben-Harari et al., 2017). Therefore, there is a critical need for further research into new drugs that are safer for patients yet still effective against the parasite.
To find the proper treatment against toxoplasmosis it is necessary to understand the molecular characteristics of the T. gondii biological cycle. The parasite's sexual phase occurs exclusively inside the feline intestine while the asexual phase occurs inside any other warm-blooded animal, including humans. There are two stages of asexual development: rapidly replicating tachyzoites responsible for acute illness, and latent bradyzoites responsible for chronic infection (Dubey et al., 1970). Tachyzoites invade any nucleated cell replicating every 5–7 h inside a specialized parasitophorous vacuole. This high replication rate is responsible for acute illness symptoms since exponential growth causes cell lysis repeatedly (Radke et al., 2001). The fast proliferation of tachyzoites could produce a replicative stress that leads to DNA damage. This was suggested by several in vitro studies where untreated tachyzoites displayed basal levels of H2A.X phosphorylation (γH2A.X), an early marker of DNA damage (Dalmasso et al., 2009; Nardelli et al., 2013) T. gondii also possesses functional pathways to repair DNA damage, such as Homologous Recombination Repair (HRR) and Non-Homologous End Joining (NHEJ) (Fenoy et al., 2016). In addition, topoisomerase I and topoisomerase II are essential genes for HRR (Angel et al., 2020), making them attractive drug targets.
Topoisomerase I (TOP1) and II (TOP2) are enzymes that solve torsional stress and supercoiled structures associated with DNA replication, recombination, transcription, chromosome condensation, and DNA damage repair pathways (Wang 2002). TOP1 cuts one of the DNA strands to allow the uncut strand to pass through the single strand break reverting the supercoiling. The cut strand is then re-ligated. TOP2 cleaves both strands using ATP and Mg++ creating a G-segment (gate) and T-segment (transported). The T segment is then passed through the cleaved G segment, the tension is released, and the segments are then re-ligated (Jain et al., 2017).
Topotecan and 10-hydroxycamptothecin (HCPT) are derivatives from camptothecin (CPT), a DNA topoisomerase I inhibitor isolated from Camptotheca acuminate (Li-Weber 2009). These compounds trap TOP1 and DNA, interrupting the re-ligation of the DNA strands, thus leading to DNA damage and afterwards activation of DNA damage response (DDR) pathways (Pommier 2006). Previous results have shown that the level of γH2A.X increases in tachyzoites treated with camptothecin (CPT), but this increase was not observed when co-administered with KU-55933, an inhibitor of the master Ataxia-telangiectasia mutated (ATM) kinase that acts in double strand break (DSB) (Munera Lopez et al., 2019). However, CPT is toxic for the host cell (Adeyemi et al., 2019; Munera Lopez et al., 2019). In contrast, topotecan has been approved by the FDA to treat various types of cancer (Jain et al., 2017) and HCPT was shown to be less toxic and more active than CPT with promising results in models of melanoma pulmonary metastases in vivo (Hu et al., 2011).
In this work, we study the effect of topotecan and HCPT on DDR and tachyzoite replication to evaluate their use as repurposed drugs against T. gondii. For this purpose, we used two different strains: RHΔHXGPRT, which possesses both NHEJ and HRR pathways to repair DSBs, and RHΔKU80, which is a null mutant for the KU80 gene that is unable to repair DNA damage through NHEJ, leaving only the HRR pathway (Fox et al., 2009; Huynh and Carruthers 2009). These two strains allowed us to compare the drug's effect in parasites with differing capacity to repair DNA. Our results indicate that topotecan and HCPT affect parasite replication in a concentration-dependent manner. The levels of γH2A.X show significant differences by fluorescence intensity under treatment conditions. Regarding the distribution of the cell cycle, topotecan did not exhibit detectable differences, while HCPT caused a significant arrest in the S phase. Furthermore, we demonstrated that γH2A.X is not present in extracellular tachyzoites, yet is present in untreated intracellular tachyzoites, indicating that T. gondii has a basal DNA replication stress. These findings provide new insights into DNA repair pathways as a potential therapeutic target in apicomplexan parasites.
2. Materials and methods
2.1. Parasite culture
Parasites of RHΔHXGPRT (Donald and Roos 1998) or RHΔKU80 (Huynh and Carruthers 2009) strain, were cultured in vitro. hTERT (BD Biosciences) fibroblast monolayers (Farwell et al., 2000) were infected with tachyzoites and incubated in Dulbecco's modified Eagle medium (DMEM, GIBCO) supplemented with 1% fetal bovine serum, penicillin (100 UI/ml; GIBCO), and streptomycin (100 μg/ml; GIBCO) at 37 °C and 5% CO2.
2.2. Primary antibody source
Rabbit anti-T. gondii γH2A.X and H2A.X antibodies were obtained from a H2A.X C-terminal peptide either phosphorylated at serine 120 or not, respectively (Contreras et al., 2021). Rabbit anti-SAG1 antibody was used as described before (Stasic et al., 2019). Murine anti-SAG antibody was purchased from INVITROGEN (MA5-18,268).
2.3. Cell viability analysis
MTT SIGMA (M2128-1G) (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assay was used to assess cell viability in response to various treatments. In this study, hTERT cells were cultured in 96-well plates until reaching 80% confluence, then treated with different concentrations of topotecan (200–0.0015 μM) and HCPT (80–0.0012 μM). After 20 h of incubation the medium was replaced for 100 μl of a MTT solution (5 mg/ml). The MTT compound is reduced by living cells, producing a violet-colored product (formazan), while dead cells cannot carry out this conversion. Then, the formazan crystals were solubilized with DMSO and the absorbance at 540 nm was measured on a microplate reader (Sinergy H1), indicating the number of viable cells present.
2.4. Crystal violet assay
Crystal violet assay was performed as previously described (Feoktistova et al., 2016) with some modifications. Briefly, hTERT cells were seeded on 24 well culture plates. When reaching 80% confluence, different concentrations of topotecan or HCPT were added within the media for 20 h. Then the medium was discarded and replaced by ethanol 70% to fix the cells at −20 °C for 10 min. After the fixation time the cells were incubated with Crystal violet 0.5% for 5 min at room temperature; the colorant was discarded, and the excess washed with PBS. Photos were obtained with Discovery V2.0 SteREO Zeiss at 422.0x.
2.5. DNA damage
T. gondii tachyzoites were used to infect hTERT monolayers for 1 h. After invasion, cells were washed with PBS and intracellular tachyzoites were treated with topotecan (Topo, Sigma-Aldrich Argentina, T2705), 10-hydroxycamptothecin (HCPT, BML-GR316-0020), or hydroxyurea (HU, Sigma-Aldrich Argentina, H8627-5G), for 20–24 h at different concentrations in each specific assay. As control, 0.1% DMSO was used.
2.6. Replication assay
Replication assay was carried out to study the effect of the drugs on the parasites. Monolayers of hTERT cells were grown on slides inside 24-well culture plates and infected with 105 RHΔKU80 or RHΔHXGPRT parasites (1 tachyzoite/cell). After 1-h incubation, cells were washed with PBS and incubated for 20 h with DMSO (control), topotecan or HCPT in different concentrations. After this period of time, cells were fixed with 100% methanol for 7 min at −20 °C and immunofluorescence staining (IFA) was performed. The number of parasites per vacuole in 100 randomly selected fields was counted. Data are presented as the mean number of tachyzoites per vacuole, after normalizing to control and transformation to semi-log scale [x = log(x)]. Statistical analysis was performed by GraphPad Prism8 using a non-linear regression parameter-dose-response inhibition-log (inhibitor) vs a normalized response-variable slope and the IC50 was obtained.
2.7. Immunofluorescence assay (IFA)
Slides of hTERT infected cells with treatments specified in each assay were blocked with 1% BSA for 30 min, then incubated with primary antibody SAG1 (mouse 1:100, Invitrogen) or anti-γH2A.X (rabbit 1:200) over-night (o.n.) at 4°C. Subsequently, slides were washed several times with PBS and the secondary antibody Alexa Fluor Goat anti-mouse 488 (Invitrogen) or Alexa Fluor Goat anti-rabbit 594 (Invitrogen) were added for 1 h at room temperature. Finally, several washes with PBS were performed and the slides were mounted with Mowiol, with or without DAPI to visualize the cell nuclei (according to the assay). A Carl Zeiss Axio Imager. M2 Microscope (Germany) with a Plan-Apochromat 63x/1.40 Oil M27 objective and a 2.8-megapixel monochrome Zeiss 503 digital video camera was used.
2.8. Plaque assay
hTERT cells were seeded on 24 well culture plates until reaching 80% confluency. They were then infected with 20 RHΔHXGPRT or RHΔKU80 total parasites and allowed to invade for 2 h. Subsequently, the drugs topotecan (1, 2, 6 and 12 μM) or HCPT (0.5, 1, 2 and 4 μM) were added, and the cells and parasites were incubated for 7 days at 37 °C. At the end of the incubation period, they were fixed with 70% ethanol at −20 °C and stained with Crystal violet 0.5% for 5 min at room temperature; the colorant was discarded. Photos were obtained with Discovery V2.0 SteREO Zeiss at 422.0x. Lysed areas were calculated using Image J software 1.53t (Fiji) and results were plotted and statistically analyzed using GraphPad Prism8, one-way ANOVA, multiple comparisons.
2.9. Cell cycle analysis
hTERT cells were grown in 6-well plates and when confluent they were infected with 106 RHΔKU80 tachyzoites per well. They were treated with different concentrations of topotecan (45 or 90 μM), HCPT (12 or 24 μM), hydroxyurea (HU 4 mM), or 0.1% v/v DMSO for 6 h. Subsequently, the plates were washed with PBS and the cells were collected with scrapers. Next, tachyzoites were forced out the cells by passage through different sized needles. Parasites were filtered using a 3 μm polycarbonate membrane (GAMAFIL). Purified parasites were centrifuged at 2000 RPM for 10 min, washed with PBS, and fixed with 70% ethanol for 24 h at −20 °C. The samples were then centrifuged and washed with PBS supplemented with 2% FBS. Then parasites were resuspended in 1 ml PBS supplemented with 180 μg/ml RNase and incubated for 10 min at 37 °C. Finally, they were incubated with propidium iodide (0.5 mg/ml) for 10 min before measuring on the BD FACS Calibur flow cytometer. Results were analyzed with FlowJo10.
2.10. Fluorescence intensity
Replication assay and IFA were performed as explained before. IFA was carried out using anti-SAG1 and anti-γH2A.X primary antibodies, and 20 images per slide were obtained. For each image, ImageJ software 1.53t (Fiji) was used to measure the fluorescence intensity generated by the anti-γH2A.X antibody. Results were statistically analyzed using GraphPad Prism8, Kruskal-Wallis tests, and plotted using Rstudio software (version March 1, 1093).
2.11. Extracellular assay
For this assay we used RHΔHXGPRT or RHΔKU80 extracellular parasites. The tachyzoites were treated as follows: 3 × 105 extracellular parasites were incubated at 37 °C for 4 h with DMSO 0.1 %, or HCPT (12 μM) or topotecan (50 μM) or phleomycin (50 μM). Extracellular parasites were fixed in slides with PAF 4% for 20 min at room temperature, then washed using PBS. IFA was performed as explained before using anti-SAG1 (mouse 1:100, Invitrogen), anti-H2A.X (rabbit 1:2000) and anti-γH2A.X (rabbit 1:200) primary antibodies. Data was analyzed with GraphPad Prism8.
2.12. Statistics
Data were expressed as mean ± SD from three to four independent experiments. Data variations were analyzed with GraphPad Prism8 software, using Student's t-test (*p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001), Kruskal Wallis test and Dunn's multiple comparisons test (****p < 0.001) and one-way ANOVA and Dunnett's multiple comparison test according to the assay.
3. Results
3.1. Impact of topotecan and HCPT on human host cells and tachyzoites
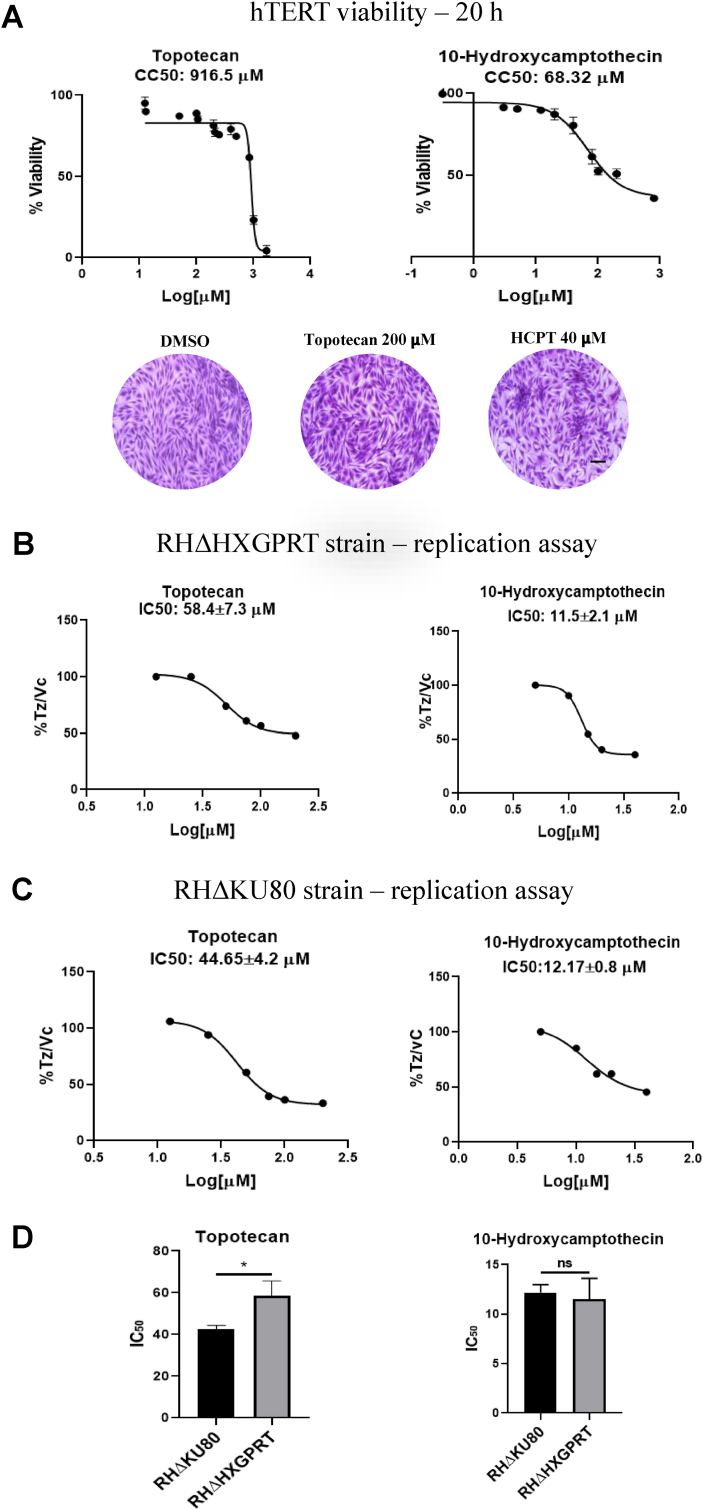
To determine the working concentrations for our study with topotecan and HCPT, we performed a cell viability assay using MTT to assess the impact of the drugs on human host cells (hTERT) after 20 h treatment. The CC50 for topotecan and HCPT in these conditions were 916.5 μM and 68.32 μM, respectively (Fig. 1A, Table 1). In order to detect host cell alterations under treatment, we analyzed the impact of these drugs at 24–48 h (Fig. S1). 200 μM topotecan affected hTERT cellular morphology after 36 h of treatment. hTERT cells lost their contact points leaving gaps between them, which intensify significantly after 48 h. Clusters of hTERT cells were observed in 40 μM HCPT, indicating cell stress. Therefore, to study the effect on parasites while avoiding damage to host cells, we used concentrations below 200 μM topotecan and 40 μM HCPT for 20 h.
Fig. 1.
Impact of topotecan and HCPT on hTERT cells and tachyzoites RHΔHXGPRT or RHΔKU80. (A) MTT assay in hTERT. Cells were treated at different concentrations of the drugs tested at 20 h, then the MTT assay was performed to analyze cell viability. CC50 values obtained are indicated above. Concentrations below topotecan 200 μM and HCPT 40 μM were used in the rest of the experiments. These concentrations were also used for the images shown below, where cells were stained with Crystal Violet. (B) and (C) Replication assays were carried out using RHΔHXGPRT or RHΔKU80 strains, as explained in Materials and Methods. Drugs were administered at concentrations less than 200 μM for topotecan (200, 100, 75, 50, 25 and 12.5 μM) and less than 40 μM for HCPT (40, 20, 15, 10, 5 μM) for 20 h. Subsequently, IFA was performed using the antibody against SAG1. The number of parasites per vacuole was counted for each concentration in 100 randomly chosen fields. The decrease in tachyzoites per parasitophorous vacuole (Tz/PV) as concentrations increase is plotted using GraphPad Prism 8 and IC50 was calculated. IC50s indicated above the graphs are the mean of three replicates plus SD. (D) IC50 comparison between strains. There is a significant difference between RHΔHXGPRT and RHΔKU80 exposed to topotecan. HCPT IC50 comparison did not show any significant difference. Statistical analysis was performed by t-test (*p < 0.05). (A), (B) and (C) show one representative of three independent assays, performed in triplicate. Scale bar 10 mm.
Table 1.
Calculation of the selectivity index for topotecan and 10-hydroxycamptothecin.
| Compound | T. gondii STRAIN | CC50 HTERT cells (μM) | IC50 T.gondii(μM) | Selectivity Index (SI) |
|---|---|---|---|---|
| Topotecan | RHΔHXGPRT | 916.5 | 58.4 | 15.7 |
| Topotecan | RHΔKU80 | 916.5 | 44.6 | 20.5 |
| 10-hydroxycamptothecin | RHΔHXGPRT | 68.3 | 11.5 | 5.9 |
| 10-hydroxycamptothecin | RHΔKU80 | 68.3 | 12.2 | 5.6 |
aThese values correspond to 20 h exposure.
To study the effect of these drugs in T. gondii, we carried out replication assays on intracellular RHΔHXGPRT (intact HRR and NHEJ pathways) and RHΔKU80 (only HRR pathway) parasites in the presence of topotecan or HCPT (Fig. 1B, C, S2). Topotecan treatment affected tachyzoite replication at IC50 58.4 μM for RHΔHXGPRT and 44.65 μM for RHΔKU80. By contrast, HCPT affected tachyzoite replication at IC50s 11.5 μM and 12.17 μM for RHΔHXGPRT and RHΔKU80, respectively. These results indicate that RHΔKU80 parasites were significantly more sensitive to topotecan than RHΔHXGPRT, while both T. gondii lines showed similar sensitivity to HCPT. Table 1 displays the CC50 and IC50 values obtained under these experimental conditions, along with the calculated selectivity index (SI).
In order to confirm anti-T. gondii effect of these genotoxic drugs, plaque assays were performed. To note, when cells were exposed to longer times (96 h) at these concentrations, cell viability dropped to around 50% (data not shown). Consequently, we conducted plaque assays to explore the impact of these drugs over extended exposure periods, using lower concentrations to prevent cell damage. These experiments revealed a significant reduction in plaque areas that correlated with concentration, reinforcing the observed anti-parasitic effects in the replication assays (Fig. S3).
3.2. Evaluation of DNA damage caused by topotecan and HCPT via fluorescence intensity measurements
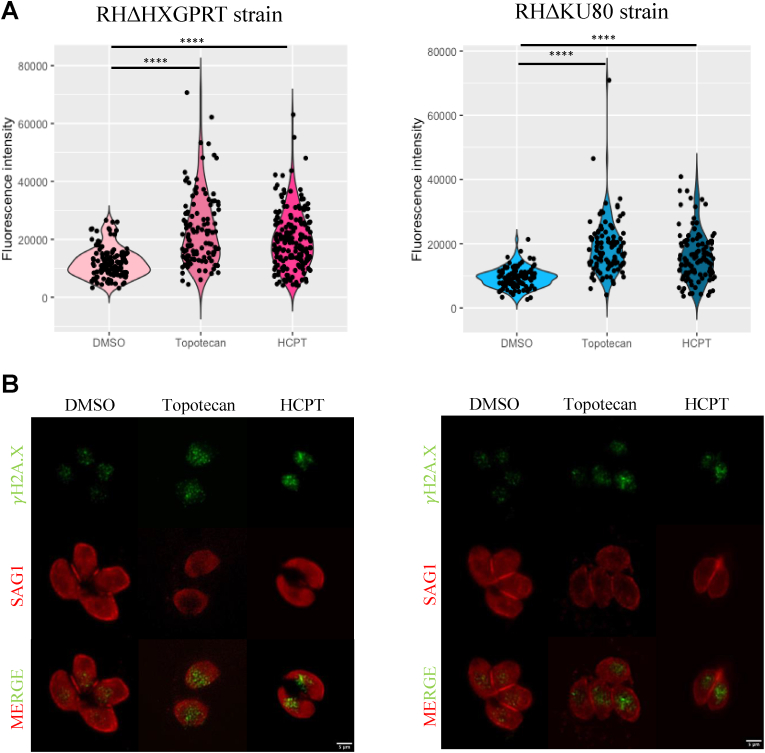
Topotecan and HCPT are genotoxic drugs used in cancer to induce DNA damage by trapping TOP1 and DNA (Pommier 2006). One of the first sensors of DSB damage is the phosphorylation of histone H2A.X, denoted as γH2A.X (Kuo and Yang 2008; Angel et al., 2020). To study if topotecan and HCPT are exerting their anti-T. gondii effect through DSB damage, we analyzed changes in γH2A.X levels. When performing a Western blot analysis of the parasites treated with the drugs, results were inconclusive (data not shown), therefore, we decided to use IFA where the γH2A.X mark in intracellular tachyzoites is clearly observed in parasite nuclei. We infected cells with tachyzoites of RHΔHXGPRT or RHΔKU80 strains and treated them with the drugs for 20 h, at the IC50 concentrations. Subsequently, IFA was performed using the γH2A.X antibody, which selectively detects the phosphorylated state of the H2A.X histone. In this way we successfully visualized the DNA damage foci, as shown in Fig. S4.
In infected cultures treated only with the vehicle control (DMSO), we detected the γH2A.X mark in 100% of the analyzed vacuoles (Fig. 2), indicating significant baseline DSB damage in proliferating tachyzoites. Despite this basal DSB damage, drug-treated intracellular tachyzoites exhibited an increase in fluorescence intensity in both strains compared to vehicle controls, suggesting increased DSB damage. Furthermore, we noticed a large variability in the fluorescence intensities obtained, not only between different vacuoles, but also between parasites from the same vacuole (Fig. 2A), suggesting that individual parasites are differentially affected.
Fig. 2.
Evaluation of DNA damage induced by topotecan and HCPT through fluorescence intensity measurements. Intracellular tachyzoites were treated with topotecan or HCPT, for 20 h, at the IC50 concentrations, or DMSO (0.1%) as control. IFAs were performed using the antibody γH2A.X, and SAG1 to easily detect parasites. Fluorescence intensity was measured using ImageJ. (A) Graphs show one representative of three independent assays, performed in triplicate, and results were plotted by Rstudio. Statistical analysis was performed by Kruskal wallis test and Dunn's multiple comparisons test (****p < 0.001) (B) Representative IFA. The images show γH2A.X in green and SAG1 in red. Scale bar 5 μm.
3.3. Effect of topotecan and HCPT on the cell cycle of RHΔKU80 parasites
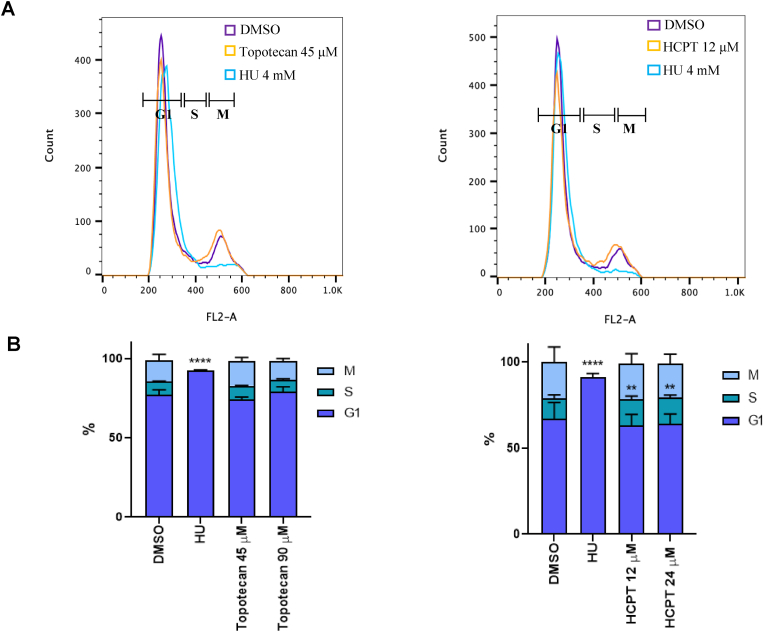
As a consequence of fork collapse after treatment with CPT derivatives, there is an interruption in the replication process that can only be restored after repairing the DSB damage, leading to cell cycle arrest (Hsiang et al., 1989; Teicher 2008). To study if this effect was observed with topotecan (45 μM [IC50] or 90 μM) or HCPT (12 μM [IC50] or 24 μM), we treated RHΔKU80 tachyzoites with the drugs for 6 h, before performing a cell cycle assay. HU (4 mM) was used as G1-arrest control (Munera Lopez et al., 2019), whereas 0.1 % v/v DMSO was used as solvent control. Subsequently, the parasites were collected and analyzed using FACS to determine the percentage of parasites in each cell cycle phase (Fig. 3A). No differences were observed between treatments with topotecan and control (DMSO) under the experimental conditions used. On the other hand, we observed that HCPT induces a modest but significant arrest of the parasites in the S phase of the cell cycle (Fig. 3B).
Fig. 3.
Analysis of RHΔKU80 tachyzoites cell cycle after treatment. (A) hTERT monolayers infected with RHΔKU80 tachyzoites were incubated with 45 or 90 μM topotecan, 12 or 24 μM HCPT, 4 mM HU and DMSO 0.1% for 6 h and propidium iodide was used to stain DNA. Tachyzoites were analyzed by FACS. One representative experiment for each drug at the lowest concentration plus controls is shown. (B) Bar graphs showing the percentage of parasites in each phase of the cell cycle. Statistical analysis was performed with one-way ANOVA and Dunnett's multiple comparison test (****p < 0.001, **p < 0.005).
3.4. Evaluation of γH2A.X and H2A.X levels in RHΔHXGPRT and RHΔKU80 extracellular tachyzoites
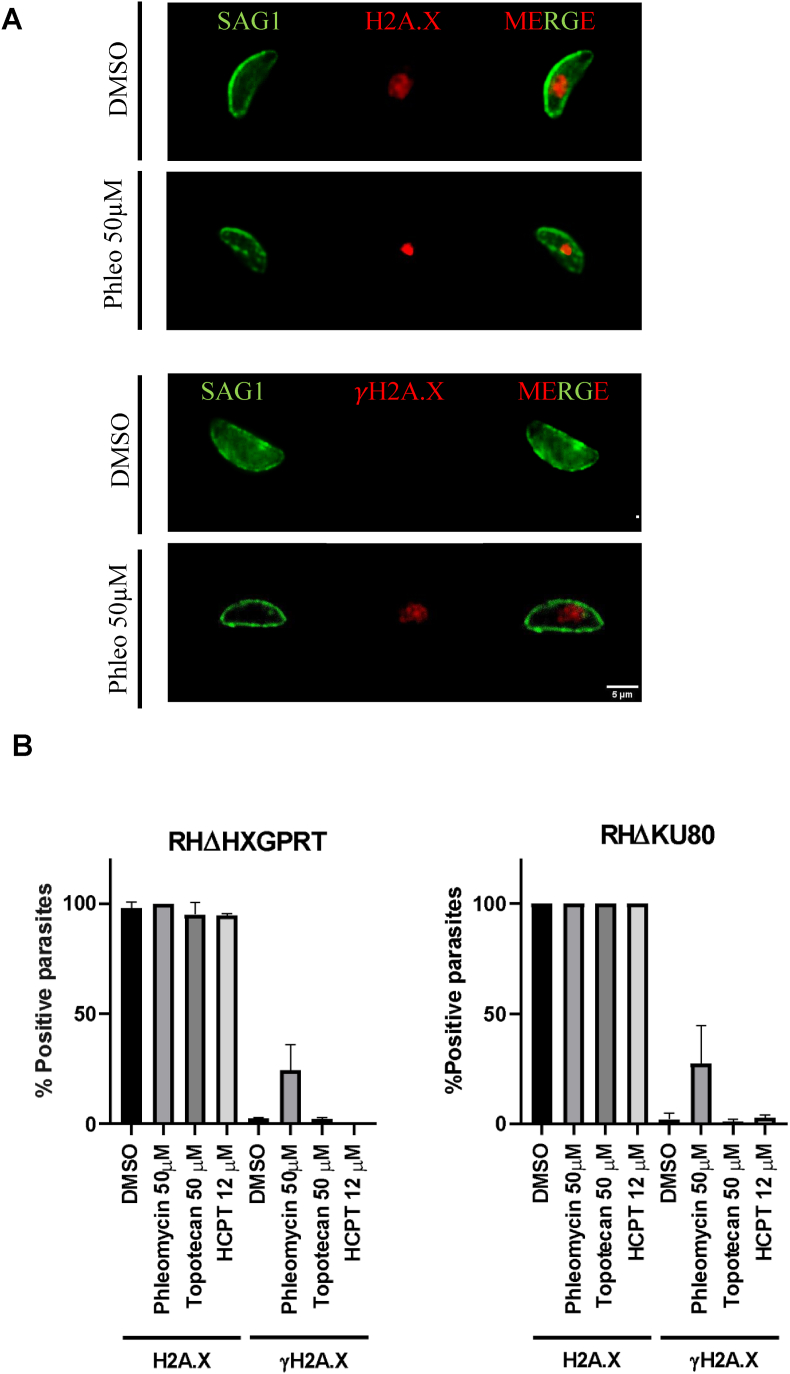
Since intracellular tachyzoites exhibit a substantial level of γH2A.X under regular growth conditions, suggesting significant DNA replicative stress, we examined the levels of γH2A.X in non-replicative extracellular tachyzoites. We treated extracellular RHΔHXGPRT and RHΔKU80 parasites with DMSO 0.1%, topotecan (50 μM), HCPT (12 μM), or phleomycin (50 μM) at 37 °C for 4 h, then IFAs were performed using γH2A.X and H2A.X antibodies. Phleomycin was used as a positive control, known for inducing extracellular DNA damage (Messina et al., 1995; Fox et al., 2009). Fig. 4 illustrates that neither strain, RHΔHXGPRT nor RHΔKU80, exhibited the γH2A.X mark under any treatment conditions except phleomycin, which showed a clear γH2A.X signal. However, all T. gondii lines displayed a positive H2A.X signal. In Fig. 4A, DMSO only is shown as control, and phleomycin to show the mark observed and quantified in Fig. 4B. Staining observed in parasites treated with topotecan and HCPT are similar as shown for DMSO. The loss of γH2A.X in extracellular non-replicative tachyzoites suggests that the levels of γH2A.X in intracellular parasites may be related to basal DSB damage, likely due to replicative stress (Dalmasso et al., 2009).
Fig. 4.
Evaluation of γH2A.X and H2A.X levels in extracellular tachyzoites. (A) Extracellular tachyzoites of RHΔHXGPRT or RHΔKU80 strains were treated with DMSO 0.1%, topotecan (50 μM), HCPT (12 μM) or phleomycin (50 μM) at 37 °C for 4 h. Phleomycin was used as a positive control for the generation of extracellular damage. Subsequently, tachyzoites were washed, fixed with 4% PAF, and IFA was performed. Anti-SAG1 mouse, anti-H2A.X rabbit and anti-γH2A.X rabbit were used as primary antibodies. The figure shows one representative image obtained for each antibody H2A.X and γH2A.X (red) and Sag1 (green). γH2A.X mark was not detected with topotecan and HCPT drugs, as well as DMSO (image shown) but we obtain positive marks in parasites treated with phleomycin (phleo). However, in all conditions H2A.X signal was observed. Scale bar 5 μM. (B) H2A.X and γH2A.X positive tachyzoites were quantified under different conditions and plotted using GraphPad software.
4. Discussion
T. gondii tachyzoites exhibit a high replication rate that can result in replication stress and the induction of DSBs. In this regard, the gene TOP1 has been identified as an essential component in the HRR DNA damage pathway, as reported before (Angel et al., 2020). Consequently, TOP1 represents a promising target for interfering with parasite replication. Little exploration has been conducted on T. gondii's DDR and genotoxic drugs primarily because of the lack of established DNA damage models. In this work, we were able to develop a DSB type DNA damage model and demonstrated the existence of basal DNA damage within the replicative form, which may be attributed to DNA replicative stress. Additionally, we introduced two novel genotoxic drugs, topotecan and HCPT, both derivatives of CPT.
While previous studies (Munera Lopez et al., 2019) have demonstrated the sensitivity of tachyzoites to CPT, a TOP1 inhibitor, this drug also exerts a significant impact on human host cells (Adeyemi et al., 2019; Munera Lopez et al., 2019). Thus, we investigated the effects of derivatives of CPT: topotecan and HCPT, the first approved by the FDA for cancer treatment and the second being a natural product under investigation in clinical trials (Angel et al., 2020), in replicative tachyzoites of the RHΔHXGPRT and RHΔKU80 strains. Replication and plaque assays showed that both drugs exert an anti-T. gondii effect at IC50 values below concentrations that are cytotoxic to host cells. The RHΔKU80 strain lacks the ku80 gene, therefore has no functional NHEJ pathway to repair DSB (Fox et al., 2009). Based on the replication assays, where both strains have similar IC50 values against HCPT would indicate that the DNA damage generated by this drug is mainly repaired by HRR, in agreement that this pathway is required for repair during the late S phase (Mao et al., 2008). However, RHΔKU80 displayed greater sensitivity to topotecan, indicating a potential reliance on the NHEJ pathway for repairing DSB damage induced by this drug. DNA damage caused by topotecan, possibly extending beyond S-phase (Feeney et al., 2003), suggests a need for repair in G1 phase, which could explain the observed differences. Nonetheless, the distinct impact was not evident in the plaque assays, possibly due to the considerable heterogeneity in plaque areas observed in this experiment. The cell cycle assay, as described below, appears to align with the results from the replication assay. However, it is important to acknowledge that the observed effects are subtle and only indicative of a certain trend.
Phosphorylation of H2A.X at the C-terminal serine (γH2A.X) indicates the presence of DSB damage and activation of DDR (Chanoux et al., 2009; Podhorecka et al., 2010). We observed a significant increase in γH2A.X intensity when intracellular tachyzoites were treated with topotecan or HCPT versus the control, indicating DSB generation beyond basal damage by these drugs. In a similar way, we observed a mild (HCPT) or no effect (topotecan) on cell cycle arrest. In previous reports studying cancer cell lines, topotecan and HCPT have been associated with arrest at the G1 or S phase of the cell cycle (Feeney et al., 2003). Our results with topotecan can potentially be attributed to its varying impact depending on the specific phase of the cell cycle in which the cell is situated (Feeney et al., 2003). Considering that our study involves a non-synchronized population, the drug's effect may vary among individual parasites. As a result, the detection of a significant effect solely attributable to topotecan during DNA replication process becomes more intricate. On the other hand, HCPT caused a moderate, although significant arrest of the parasites in the S phase. These results would indicate that this is the phase in which the DNA damage occurs, therefore preventing the parasite cell cycle to continue until it is repaired, similar to the effect in cancer cells (Hu et al., 2011). In line with this, the outcomes from the replication assays, which indicate that RHΔKU80 parasites are more sensitive than RHΔHXGPRT to topotecan but not to HCPT, further support the action in S phase of the former compound, causing a DSB damage that is repaired by HRR. Based on these results, it is possible that the drugs, at least HCPT, induce DSB damage as observed for other organisms, whose modest impact may be masked by the high baseline DDR in replicating tachyzoites.
Interestingly, extracellular tachyzoites showed no γH2A.X signal, either under negative control conditions or treated with topotecan or HCPT. However, treatment with phleomycin induced DNA damage in extracellular tachyzoites, evidenced by the γH2A.X mark (Vitor et al., 2020). This would corroborate the existence of substantial replicative DNA stress occurring during the tachyzoite cell cycle, thereby generating the baseline DSB damage observed by the presence of γH2A.X in intracellular tachyzoites. However, T. gondii successfully overcomes this adverse effect of rapid replication, implying a great capacity to resolve DSB damage. Absence of γH2A.X signal in extracellular parasites, in comparison to the intracellular stadium, suggests proficient damage resolution. Notably, T. gondii has preserved these different DNA repair pathways (Smolarz et al., 2014) while other parasites, even some in the same phylum, like Plasmodium, only retain HRR to solve DSBs (Kirkman et al., 2014). Plasmodium falciparum developed a complex DNA repair system to maintain its genomic integrity, which remains poorly understood. A role of the nuclease PfAlba3 on the DNA-damage response, which could have an effect in pathogenicity, has been recently identified (Banerjee et al., 2023). In T. gondii, many of the NHEJ or HRR associated proteins are essential for the lytic cycle (Angel et al., 2020). This supports a relevant role for the DDR in the high replicative tachyzoite under normal conditions.
Our findings indicate that the sensitivity to topotecan and HCPT is likely linked to DSB damage, although their effects are not as compelling as in other systems. It is important to mention that previous studies have demonstrated that camptothecin derivatives in parasites such as T. brucei and T. cruzi could have different effects compared to cancer cell lines. These differences include higher IC50 or the absence of a significant DNA damaging effect in experiments at the population level (Lacombe et al., 2014). These observations suggest potential disparities between human and T. gondii TOP1. The development of specific drugs targeting parasite infections becomes necessary to ensure safe and targeted therapy against the parasite while minimizing harm to the host cell. However, it is also important to note that the limited efficacy observed could be also attributed to efficient DDR mechanisms employed by T. gondii. Therefore, combining genotoxic drugs with compounds that disrupt DDR, as previously observed, could enhance and improve the anti-toxoplasmic effect of HCPT and/or topotecan (Munera Lopez et al., 2019).
4.1. Conclusion
Our results reinforce the importance of studying DNA damage repair mechanisms in T. gondii to identify potential targets for inhibition that would prevent the parasite from continued replication. The high levels of basal γH2A.X in proliferating parasites suggest these parasites harbor a robust DNA repair system that may require a cocktail drug therapy to neutralize effectively. Identifying specific genotoxic drugs that could be used as effective agents against parasites, in combination with DDR-altering compounds, could contribute to the development of a safe and efficacious treatment against toxoplasmosis.
Author contributions
Experiments were designed by SA and LV. CC and AS carried out the experiments and the analysis, as well as writing the first draft of the manuscript, equally. AG contributed with technical support and preparation of media, as well as the cell cycle experiments. SA, LV, and WJS, contributed with the direction, analysis of the data, and final writing the manuscript.
Funding
This research was supported by the Ministerio Nacional de Ciencia y Tecnología (MINCyT): PICT 2021–2704 (SOA), PICT 2018 2434 (LV), PICT 2021 0169 (LV), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET): 11220210100572CO (SOA, LV) and by National Institutes of Health: NIH-NIAID 1R01AI129807 (SOA and WJS).
Data availability Statement
All data that are available from our lab and relevant to this study are included in the article. All the raw data generated are available upon reasonable request to corresponding authors.
Declaration of competing interest
All authors declare that there are no conflicts of interest.
There is no financial or non-financial assistance provided by a third party for the reported work.
There is no financial interest or relationship related to the subject matter.
Acknowledgements
CC (Fellow), AS (Fellow), AG (Technician), SA (Researcher) and LV (Researcher), are members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). WJS (Researcher) is member of Indiana University School of Medicine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2023.11.004.
Contributor Information
Sergio O. Angel, Email: sangel@intech.gov.ar.
Laura Vanagas, Email: vanagas@intech.gov.ar.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Effect of different doses of topotecan and HCPT during time on hTERT cells. hTERT cells were grown on 6 well plates and treated with different doses (25, 50, 100, 200 μM) of topotecan or (6, 12, 24, 40 μM) of HCPT for 24, 36 or 48 h. DMSO (0.1%) was used as control. After that, they were fixed and stained with crystal violet. There is uniform distribution of the monolayer without cell alterations except at 200 μm for topotecan and 40 μm for HCPT and where thinner structures (red arrows) and cumulus of cells (red circles) indicative of cellular stress. Scale bar 10 mm.
SUPPLEMENTARY FIGURE S2. A. Illustrative images that show vacuoles of 1, 2, 4, 8 parasites. Scale bar 5 μm. B. Representative IFAs obtained during the replication assay are shown in the conditions: DMSO, topotecan at 50 μM, and HCPT at 10 μM. IFA technique was carried out following the protocol detailed in the Materials and Methods section, using the primary antibody SAG1 (1:500) and the secondary antibody Alexa fluor goat anti-mouse 488 (Invitrogen). Scale bar 100 μm.
SUPPLEMENTARY FIGURE S3. Effect of topotecan and HCPT on T.gondii in longer expositions by plaque assays. Representative images (from two independent assays) of each concentration tested, along with the graph showing quantification of the areas is shown for RHΔHXGPRT (A, B) and RHΔKU80 (C, D), after 7 days of exposition to topotecan (A, C) or HCPT (B, D). Lysed areas were calculated as outlined in Materials and Methods and results were plotted and statistically analyzed using GraphPad Prism8, one-way ANOVA, multiple comparisons. *p < 0.05; ***p < 0.0005; ****p < 0.0001. Scale bar = 1 mm.
SUPPLEMENTARY FIGURE S4. Representative IFA with the γH2A.X and H2A.X antibodies. hTERT cells were grown on glass slides and infected with RHΔHXGPRT parasites for 24 h. Subsequently, cells were fixed and the IFA was performed. The primary antibodies used were γH2A.X (rabbit 1:200), H2A.X (rabbit 1:500) and SAG1 (mouse 1:500). Secondary antibodies Alexa fluor goat anti-mouse 488 and anti-rabbit 594 (Invitrogen) were used. Scale bar 5 μm.
References
- Adeyemi O.S., Atolani O., Awakan O.J., Olaolu T.D., Nwonuma C.O., Alejolowo O., Otohinoyi D.A., Rotimi D., Owolabi A., Batiha G.E. In vitro screening to identify anti-toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J. Biol. Med. 2019;92:369–383. [PMC free article] [PubMed] [Google Scholar]
- Angel S.O., Vanagas L., Ruiz D.M., Cristaldi C., Saldarriaga Cartagena A.M., Sullivan W.J., Jr. Emerging therapeutic targets against toxoplasma gondii: update on DNA repair response inhibitors and genotoxic drugs. Front. Cell. Infect. Microbiol. 2020;10:289. doi: 10.3389/fcimb.2020.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C., Nag S., Goyal M., Saha D., Siddiqui A.A., Mazumder S., Debsharma S., Pramanik S., Bandyopadhyay U. Nuclease activity of Plasmodium falciparum Alba family protein PfAlba3. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112292. [DOI] [PubMed] [Google Scholar]
- Basavaraju A. Toxoplasmosis in HIV infection: an overview. Tropenmed. Parasitol. 2016;6:129–135. doi: 10.4103/2229-5070.190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Harari R.R., Goodwin E., Casoy J. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: a systematic review. Drugs R. 2017;17:523–544. doi: 10.1007/s40268-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanoux R.A., Yin B., Urtishak K.A., Asare A., Bassing C.H., Brown E.J. ATR and H2AX cooperate in maintaining genome stability under replication stress. J. Biol. Chem. 2009;284:5994–6003. doi: 10.1074/jbc.M806739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras S.M., Ganuza A., Corvi M.M., Angel S.O. Resveratrol induces H3 and H4K16 deacetylation and H2A.X phosphorylation in Toxoplasma gondii. BMC Res. Notes. 2021;14:19. doi: 10.1186/s13104-020-05416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso M.C., Onyango D.O., Naguleswaran A., Sullivan W.J., Jr., Angel S.O. Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family. J. Mol. Biol. 2009;392:33–47. doi: 10.1016/j.jmb.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R.G., Roos D.S. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol. Biochem. Parasitol. 1998;91:295–305. doi: 10.1016/s0166-6851(97)00210-7. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Miller N.L., Frenkel J.K. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 1970;132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell D.G., Shera K.A., Koop J.I., Bonnet G.A., Matthews C.P., Reuther G.W., Coltrera M.D., McDougall J.K., Klingelhutz A.J. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 2000;156:1537–1547. doi: 10.1016/S0002-9440(10)65025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney G.P., Errington R.J., Wiltshire M., Marquez N., Chappell S.C., Smith P.J. Tracking the cell cycle origins for escape from topotecan action by breast cancer cells. Br. J. Cancer. 2003;88:1310–1317. doi: 10.1038/sj.bjc.6600889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoy I.M., Bogado S.S., Contreras S.M., Gottifredi V., Angel S.O. The knowns unknowns: exploring the homologous recombination repair pathway in toxoplasma gondii. Front. Microbiol. 2016;7:627. doi: 10.3389/fmicb.2016.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova M., Geserick P., Leverkus M. Cold Spring Harb Protoc; 2016. Crystal Violet Assay for Determining Viability of Cultured Cells. 2016: pdb prot087379. [DOI] [PubMed] [Google Scholar]
- Flegr J. Effects of toxoplasma on human behavior. Schizophr. Bull. 2007;33:757–760. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B.A., Ristuccia J.G., Gigley J.P., Bzik D.J. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot. Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Dubey J.P., Hill D., Buchanan R.L., Gamble H.R., Jones J.L., Pradhan A.K. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food Protect. 2015;78:457–476. doi: 10.4315/0362-028X.JFP-14-328. [DOI] [PubMed] [Google Scholar]
- Hsiang Y.H., Lihou M.G., Liu L.F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- Hu W., Zhang C., Fang Y., Lou C. Anticancer properties of 10-hydroxycamptothecin in a murine melanoma pulmonary metastasis model in vitro and in vivo. Toxicol. Vitro. 2011;25:513–520. doi: 10.1016/j.tiv.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Huynh M.H., Carruthers V.B. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C.K., Majumder H.K., Roychoudhury S. Natural compounds as anticancer agents targeting DNA topoisomerases. Curr. Genom. 2017;18:75–92. doi: 10.2174/1389202917666160808125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman L.A., Lawrence E.A., Deitsch K.W. Malaria parasites utilize both homologous recombination and alternative end joining pathways to maintain genome integrity. Nucleic Acids Res. 2014;42:370–379. doi: 10.1093/nar/gkt881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L.J., Yang L.X. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- Lacombe O.K., Zuma A.A., da Silva C.C., de Souza W., Motta M.C. Effects of camptothecin derivatives and topoisomerase dual inhibitors on Trypanosoma cruzi growth and ultrastructure. J. Negat. Results Biomed. 2014;13:11. doi: 10.1186/1477-5751-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Mao Z., Bozzella M., Seluanov A., Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M., Niesman I., Mercier C., Sibley L.D. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165:213–217. doi: 10.1016/0378-1119(95)00548-k. [DOI] [PubMed] [Google Scholar]
- Munera Lopez J., Ganuza A., Bogado S.S., Munoz D., Ruiz D.M., Sullivan W.J., Jr., Vanagas L., Angel S.O. Evaluation of ATM kinase inhibitor KU-55933 as potential anti-toxoplasma gondii agent. Front. Cell. Infect. Microbiol. 2019;9:26. doi: 10.3389/fcimb.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli S.C., Che F.Y., Silmon de Monerri N.C., Xiao H., Nieves E., Madrid-Aliste C., Angel S.O., Sullivan W.J., Jr., Angeletti R.H., Kim K., Weiss L.M. The histone code of Toxoplasma gondii comprises conserved and unique posttranslational modifications. mBio. 2013;4 doi: 10.1128/mBio.00922-13. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorecka M., Skladanowski A., Bozko P. J Nucleic Acids; 2010. H2AX Phosphorylation: its Role in DNA Damage Response and Cancer Therapy. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- Radke Jay R., Striepen Boris, Guerini Michael N., Jerome Maria E., Roos David S., White Michael W. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol. Biochem. Parasitol. 2001;115:165–175. doi: 10.1016/s0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Smolarz B., Wilczynski J., Nowakowska D. DNA repair mechanisms and Toxoplasma gondii infection. Arch. Microbiol. 2014;196:1–8. doi: 10.1007/s00203-013-0944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasic A.J., Chasen N.M., Dykes E.J., Vella S.A., Asady B., Starai V.J., Moreno S.N.J. The toxoplasma vacuolar H(+)-ATPase regulates intracellular pH and impacts the maturation of essential secretory proteins. Cell Rep. 2019;27:2132. doi: 10.1016/j.celrep.2019.04.038. 21346 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B.A. Next generation topoisomerase I inhibitors: rationale and biomarker strategies. Biochem. Pharmacol. 2008;75:1262–1271. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Tenter Astrid M., Heckeroth Anja R., Weiss Louis M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitor A.C., Huertas P., Legube G., de Almeida S.F. Studying DNA double-strand break repair: an ever-growing toolbox. Front. Mol. Biosci. 2020;7:24. doi: 10.3389/fmolb.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of different doses of topotecan and HCPT during time on hTERT cells. hTERT cells were grown on 6 well plates and treated with different doses (25, 50, 100, 200 μM) of topotecan or (6, 12, 24, 40 μM) of HCPT for 24, 36 or 48 h. DMSO (0.1%) was used as control. After that, they were fixed and stained with crystal violet. There is uniform distribution of the monolayer without cell alterations except at 200 μm for topotecan and 40 μm for HCPT and where thinner structures (red arrows) and cumulus of cells (red circles) indicative of cellular stress. Scale bar 10 mm.
SUPPLEMENTARY FIGURE S2. A. Illustrative images that show vacuoles of 1, 2, 4, 8 parasites. Scale bar 5 μm. B. Representative IFAs obtained during the replication assay are shown in the conditions: DMSO, topotecan at 50 μM, and HCPT at 10 μM. IFA technique was carried out following the protocol detailed in the Materials and Methods section, using the primary antibody SAG1 (1:500) and the secondary antibody Alexa fluor goat anti-mouse 488 (Invitrogen). Scale bar 100 μm.
SUPPLEMENTARY FIGURE S3. Effect of topotecan and HCPT on T.gondii in longer expositions by plaque assays. Representative images (from two independent assays) of each concentration tested, along with the graph showing quantification of the areas is shown for RHΔHXGPRT (A, B) and RHΔKU80 (C, D), after 7 days of exposition to topotecan (A, C) or HCPT (B, D). Lysed areas were calculated as outlined in Materials and Methods and results were plotted and statistically analyzed using GraphPad Prism8, one-way ANOVA, multiple comparisons. *p < 0.05; ***p < 0.0005; ****p < 0.0001. Scale bar = 1 mm.
SUPPLEMENTARY FIGURE S4. Representative IFA with the γH2A.X and H2A.X antibodies. hTERT cells were grown on glass slides and infected with RHΔHXGPRT parasites for 24 h. Subsequently, cells were fixed and the IFA was performed. The primary antibodies used were γH2A.X (rabbit 1:200), H2A.X (rabbit 1:500) and SAG1 (mouse 1:500). Secondary antibodies Alexa fluor goat anti-mouse 488 and anti-rabbit 594 (Invitrogen) were used. Scale bar 5 μm.
Data Availability Statement
All data that are available from our lab and relevant to this study are included in the article. All the raw data generated are available upon reasonable request to corresponding authors.