Abstract
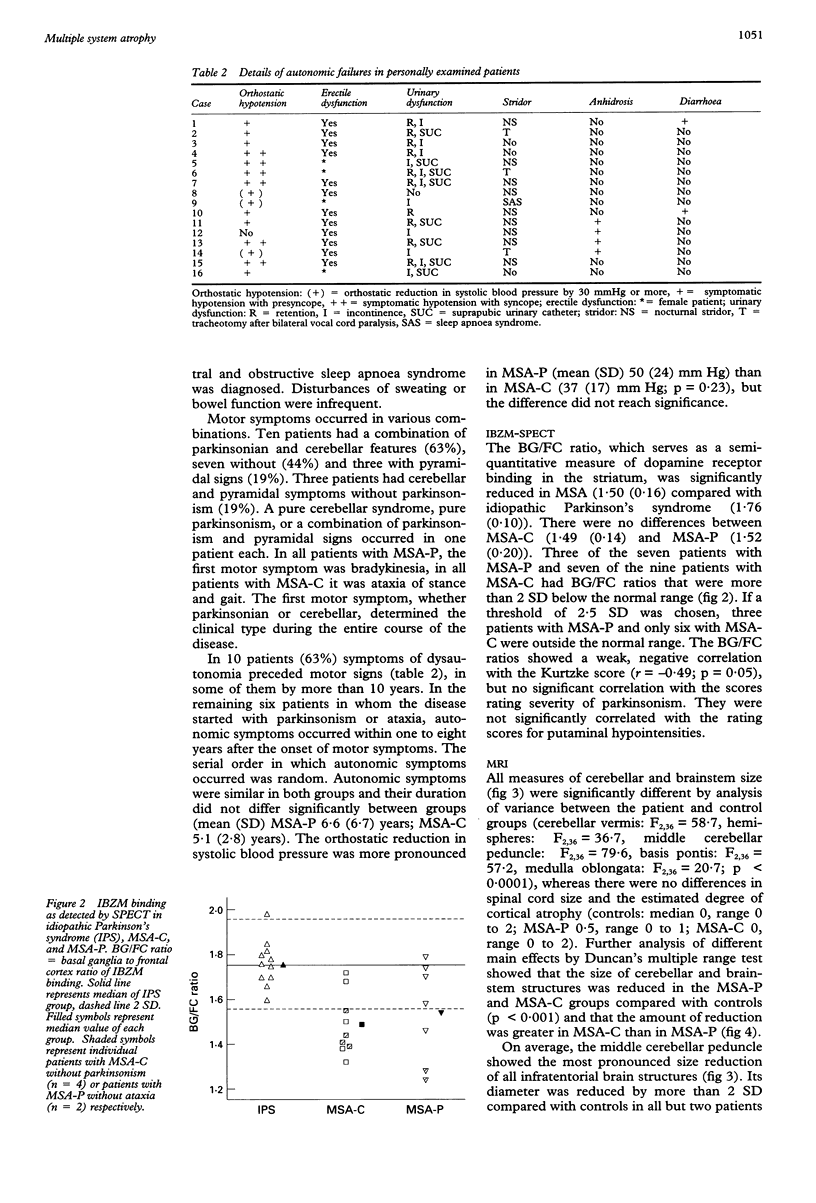
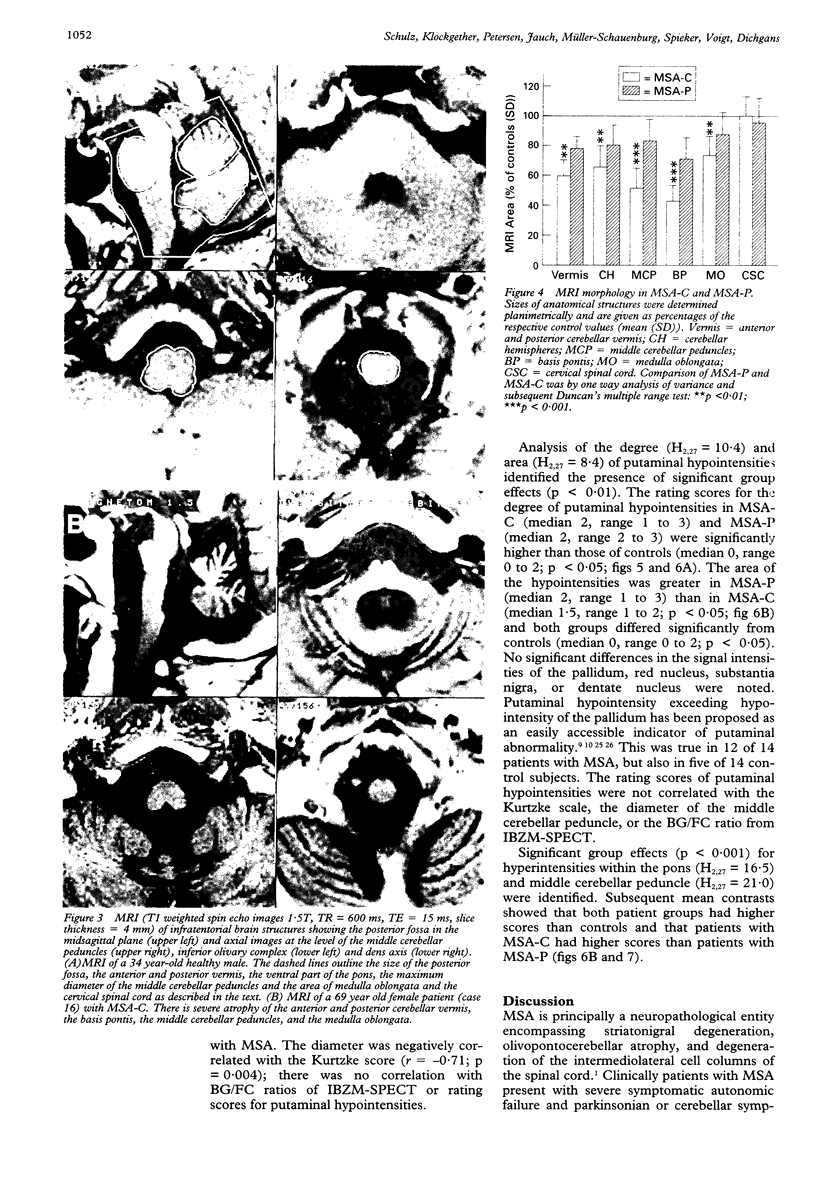
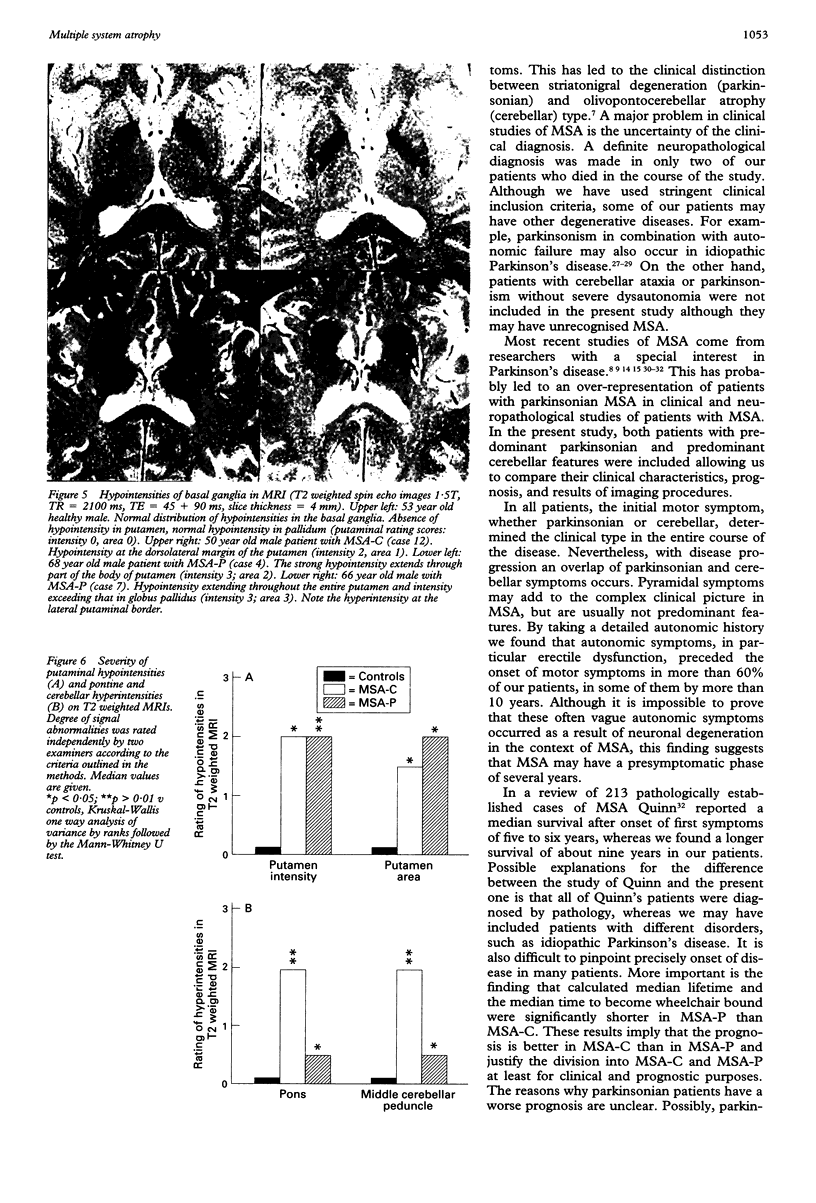
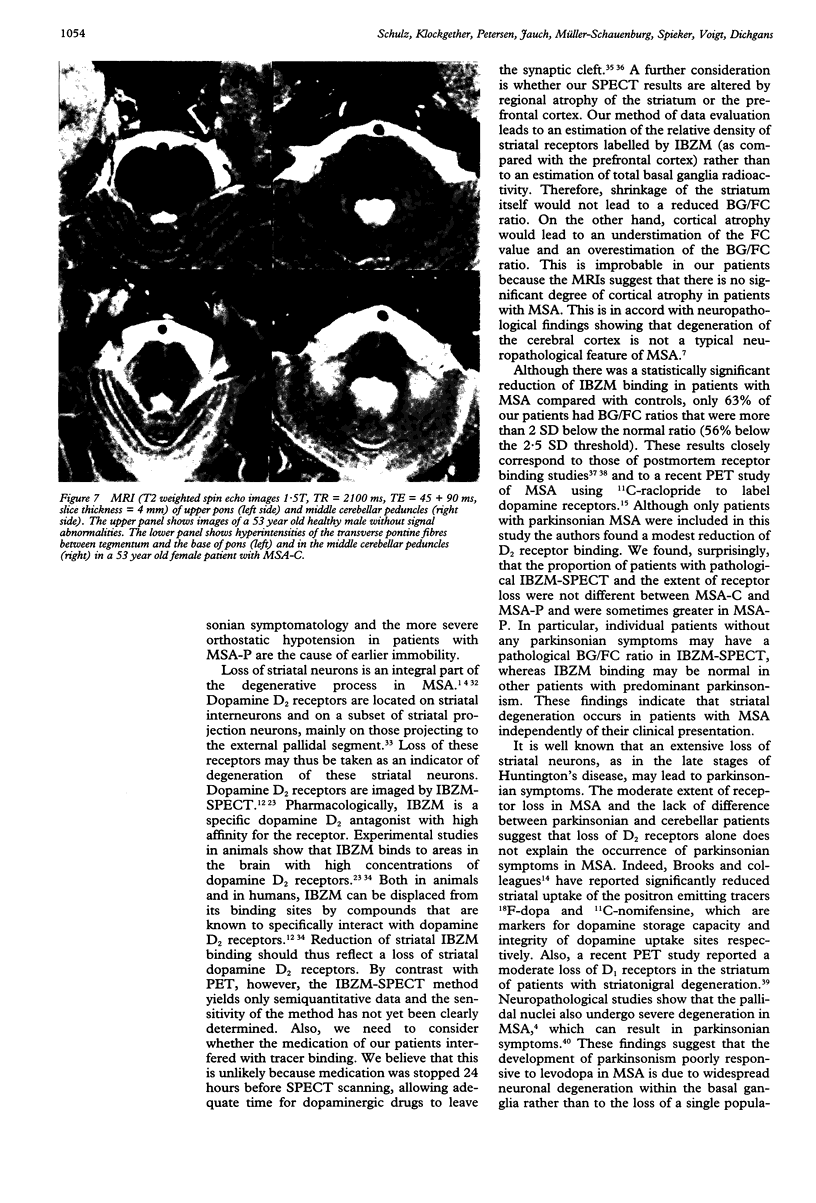
Sixteen patients with a clinical diagnosis of probable multiple system atrophy (MSA) were examined clinically by MRI and by 123I-iodobenzamide single photon emission computed tomography (IBZM-SPECT). The clinical records of another 16 patients were also analysed retrospectively. On the basis of their clinical presentation, patients were subdivided into those with prominent parkinsonism (MSA-P, n = 11) and those with prominent cerebellar ataxia (MSA-C, n = 21). Autonomic symptoms were present in all patients and preceded the onset of motor symptoms in 63% of patients. Calculated median lifetime and the median time to become wheelchair bound after onset of disease were significantly shorter for MSA-P than for MSA-C (lifetime: 4.0 v 9.1 years; wheelchair: 3.1 vs 5.0 years) suggesting a better prognosis for cerebellar patients. A significant loss of striatal dopamine receptors (below 2 SD threshold) was detected by IBZM-SPECT in 63% of the patients (56% below 2.5 SD threshold). There was no difference between patients with MSA-C and those with MSA-P in the proportion with significant receptor loss and the extent of dopamine receptor loss. Planimetric MRI evaluation showed cerebellar and brainstem atrophy in both groups. Atrophy was more pronounced in patients with MSA-C than in those with MSA-P. Pontocerebellar hyperintensities and putaminal hypointensities on T2 weighted MRI were found in both groups. Pontocerebellar signal abnormalities were more pronounced in MSA-C than in MSA-P, whereas the rating scores for area but not for intensity of putaminal abnormalities were higher in MSA-P. MRI and IBZM-SPECT provide in vivo evidence for combined basal ganglia and pontocerebellar involvement in almost all patients in this series.
Full text
PDF




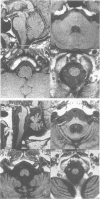
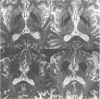
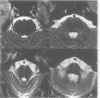
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister R., Oppenheimer D. R. Degenerative diseases of the nervous system associated with autonomic failure. Brain. 1972;95(3):457–474. doi: 10.1093/brain/95.3.457. [DOI] [PubMed] [Google Scholar]
- Brooks D. J., Ibanez V., Sawle G. V., Playford E. D., Quinn N., Mathias C. J., Lees A. J., Marsden C. D., Bannister R., Frackowiak R. S. Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol. 1992 Feb;31(2):184–192. doi: 10.1002/ana.410310209. [DOI] [PubMed] [Google Scholar]
- Brooks D. J., Luthert P., Gadian D., Marsden C. D. Does signal-attenuation on high-field T2-weighted MRI of the brain reflect regional cerebral iron deposition? Observations on the relationship between regional cerebral water proton T2 values and iron levels. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):108–111. doi: 10.1136/jnnp.52.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. J., Salmon E. P., Mathias C. J., Quinn N., Leenders K. L., Bannister R., Marsden C. D., Frackowiak R. S. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson's disease, studied with PET. Brain. 1990 Oct;113(Pt 5):1539–1552. doi: 10.1093/brain/113.5.1539. [DOI] [PubMed] [Google Scholar]
- Brücke T., Podreka I., Angelberger P., Wenger S., Topitz A., Küfferle B., Müller C., Deecke L. Dopamine D2 receptor imaging with SPECT: studies in different neuropsychiatric disorders. J Cereb Blood Flow Metab. 1991 Mar;11(2):220–228. doi: 10.1038/jcbfm.1991.53. [DOI] [PubMed] [Google Scholar]
- Delforge J., Loc'h C., Hantraye P., Stulzaft O., Khalili-Varasteh M., Mazière M., Syrota A., Mazière B. Kinetic analysis of central [76Br]bromolisuride binding to dopamine D2 receptors studied by PET. J Cereb Blood Flow Metab. 1991 Nov;11(6):914–925. doi: 10.1038/jcbfm.1991.156. [DOI] [PubMed] [Google Scholar]
- Drayer B. P., Olanow W., Burger P., Johnson G. A., Herfkens R., Riederer S. Parkinson plus syndrome: diagnosis using high field MR imaging of brain iron. Radiology. 1986 May;159(2):493–498. doi: 10.1148/radiology.159.2.3961182. [DOI] [PubMed] [Google Scholar]
- Drewe J., Mazer N., Abisch E., Krummen K., Keck M. Differential effect of food on kinetics of bromocriptine in a modified release capsule and a conventional formulation. Eur J Clin Pharmacol. 1988;35(5):535–541. doi: 10.1007/BF00558250. [DOI] [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. Striatonigral degeneration. A clinicopathological study. Brain. 1990 Dec;113(Pt 6):1823–1842. doi: 10.1093/brain/113.6.1823. [DOI] [PubMed] [Google Scholar]
- Goetz C. G., Lutge W., Tanner C. M. Autonomic dysfunction in Parkinson's disease. Neurology. 1986 Jan;36(1):73–75. doi: 10.1212/wnl.36.1.73. [DOI] [PubMed] [Google Scholar]
- Graham J. G., Oppenheimer D. R. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969 Feb;32(1):28–34. doi: 10.1136/jnnp.32.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F., Vincent D., Hauw J. J. Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol. 1988;75(5):513–518. doi: 10.1007/BF00687140. [DOI] [PubMed] [Google Scholar]
- Hoehn M. M., Yahr M. D. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Klockgether T., Schroth G., Diener H. C., Dichgans J. Idiopathic cerebellar ataxia of late onset: natural history and MRI morphology. J Neurol Neurosurg Psychiatry. 1990 Apr;53(4):297–305. doi: 10.1136/jnnp.53.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller W. C., Vetere-Overfield B., Williamson A., Busenbark K., Nash J., Parrish D. Sexual dysfunction in Parkinson's disease. Clin Neuropharmacol. 1990 Oct;13(5):461–463. doi: 10.1097/00002826-199010000-00008. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Guo Y. Z., Billings J., Xu X., Mach R. H., Blau M., Ackerhalt R. E. Preparation and biodistribution of [125I]IBZM: a potential CNS D-2 dopamine receptor imaging agent. Int J Rad Appl Instrum B. 1988;15(2):195–201. doi: 10.1016/0883-2897(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Pan S., Kung M. P., Billings J., Kasliwal R., Reilley J., Alavi A. In vitro and in vivo evaluation of [123I]IBZM: a potential CNS D-2 dopamine receptor imaging agent. J Nucl Med. 1989 Jan;30(1):88–92. [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. Magnetic resonance imaging in parkinsonism. Neurol Clin. 1992 May;10(2):405–420. [PubMed] [Google Scholar]
- Papp M. I., Kahn J. E., Lantos P. L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989 Dec;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Pastakia B., Polinsky R., Di Chiro G., Simmons J. T., Brown R., Wener L. Multiple system atrophy (Shy-Drager syndrome): MR imaging. Radiology. 1986 May;159(2):499–502. doi: 10.1148/radiology.159.2.3961183. [DOI] [PubMed] [Google Scholar]
- Quik M., Spokes E. G., Mackay A. V., Bannister R. Alterations in [3H]spiperone binding in human caudate nucleus, substantia nigra and frontal cortex in the Shy-Drager syndrome and Parkinson's disease. J Neurol Sci. 1979 Nov;43(3):429–437. doi: 10.1016/0022-510x(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Quinn N. Multiple system atrophy--the nature of the beast. J Neurol Neurosurg Psychiatry. 1989 Jun;Suppl:78–89. doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoiardo M., Strada L., Girotti F., D'Incerti L., Sberna M., Soliveri P., Balzarini L. MR imaging in progressive supranuclear palsy and Shy-Drager syndrome. J Comput Assist Tomogr. 1989 Jul-Aug;13(4):555–560. doi: 10.1097/00004728-198907000-00001. [DOI] [PubMed] [Google Scholar]
- Savoiardo M., Strada L., Girotti F., Zimmerman R. A., Grisoli M., Testa D., Petrillo R. Olivopontocerebellar atrophy: MR diagnosis and relationship to multisystem atrophy. Radiology. 1990 Mar;174(3 Pt 1):693–696. doi: 10.1148/radiology.174.3.2305051. [DOI] [PubMed] [Google Scholar]
- Schroth G., Naegele T., Klose U., Mann K., Petersen D. Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology. 1988;30(5):385–389. doi: 10.1007/BF00404102. [DOI] [PubMed] [Google Scholar]
- Schwarz J., Tatsch K., Arnold G., Gasser T., Trenkwalder C., Kirsch C. M., Oertel W. H. 123I-iodobenzamide-SPECT predicts dopaminergic responsiveness in patients with de novo parkinsonism. Neurology. 1992 Mar;42(3 Pt 1):556–561. doi: 10.1212/wnl.42.3.556. [DOI] [PubMed] [Google Scholar]
- Shinotoh H., Inoue O., Hirayama K., Aotsuka A., Asahina M., Suhara T., Yamazaki T., Tateno Y. Dopamine D1 receptors in Parkinson's disease and striatonigral degeneration: a positron emission tomography study. J Neurol Neurosurg Psychiatry. 1993 May;56(5):467–472. doi: 10.1136/jnnp.56.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokes E. G., Bannister R., Oppenheimer D. R. Multiple system atrophy with autonomic failure: clinical, histological and neurochemical observations on four cases. J Neurol Sci. 1979 Sep;43(1):59–82. doi: 10.1016/0022-510x(79)90073-x. [DOI] [PubMed] [Google Scholar]
- Stern M. B., Braffman B. H., Skolnick B. E., Hurtig H. I., Grossman R. I. Magnetic resonance imaging in Parkinson's disease and parkinsonian syndromes. Neurology. 1989 Nov;39(11):1524–1526. doi: 10.1212/wnl.39.11.1524. [DOI] [PubMed] [Google Scholar]
- Wüllner U., Klockgether T., Petersen D., Naegele T., Dichgans J. Magnetic resonance imaging in hereditary and idiopathic ataxia. Neurology. 1993 Feb;43(2):318–325. doi: 10.1212/wnl.43.2.318. [DOI] [PubMed] [Google Scholar]