Abstract
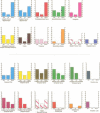
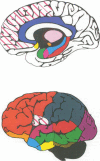
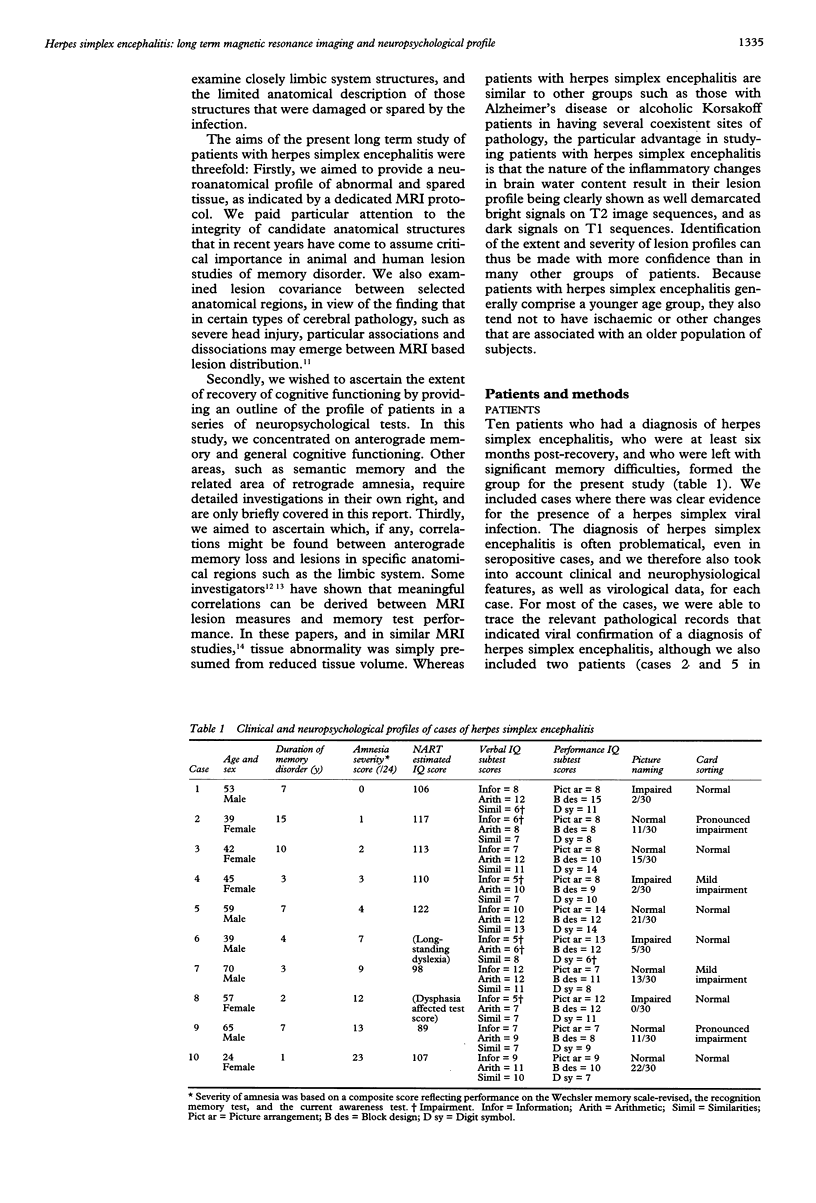
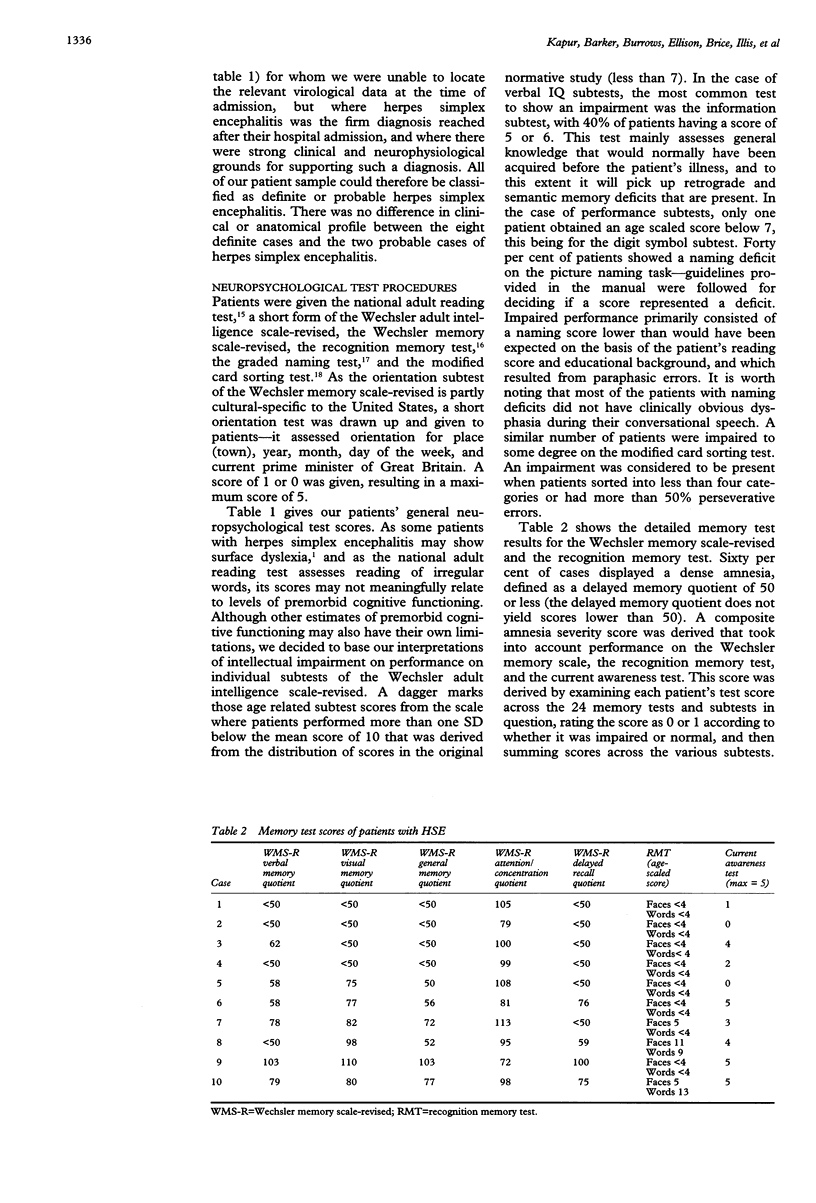
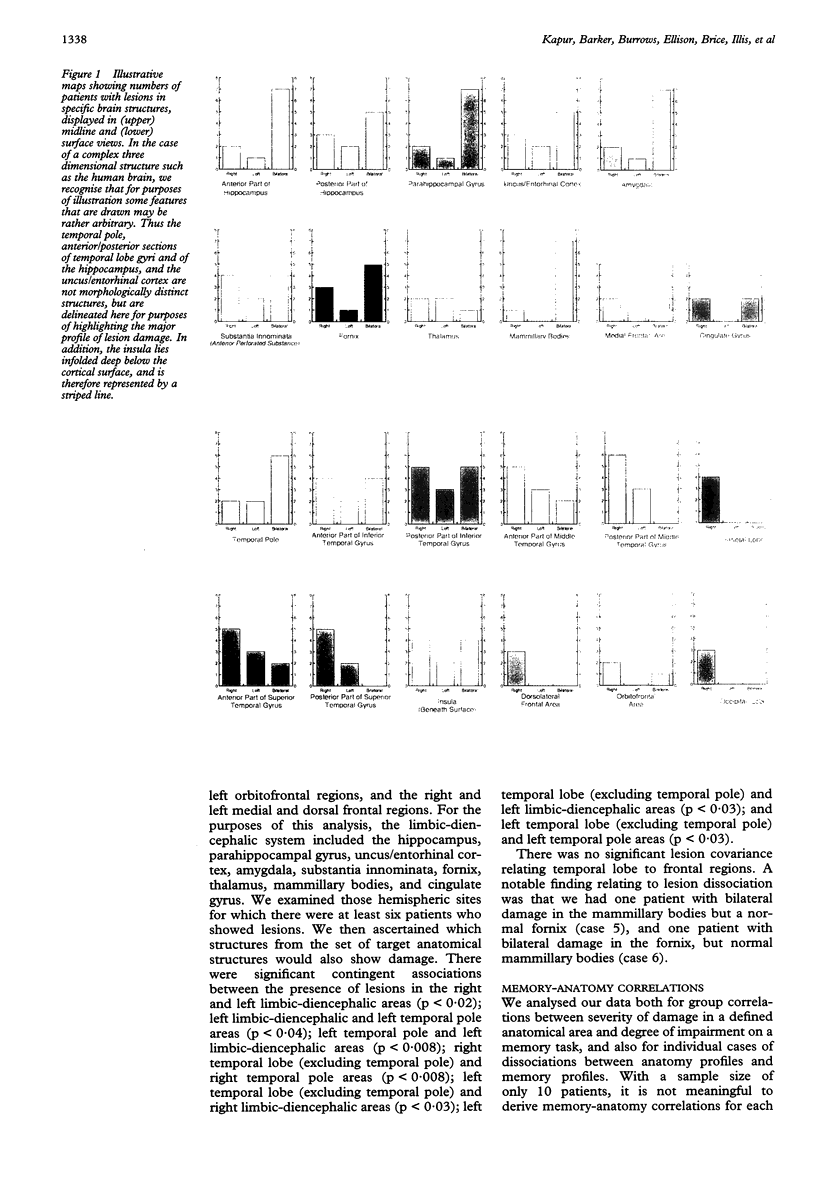
The first comprehensive in vivo documentation of the long term profile of pathological and spared tissue is described in a group of 10 patients with a diagnosis of herpes simplex encephalitis, who were left with memory difficulties as a major residual sequel of their condition. With a dedicated MRI protocol, which included high resolution images of temporal lobe and limbic system areas, data are provided on structures that have recently gained importance as anatomical substrates for amnesia. The major features of the lesion profile were: (1) unilateral or bilateral hippocampal damage never occurred in isolation, and was often accompanied by damage to the parahippocampus, the amygdala, specific temporal lobe gyri, and the temporal poles; (2) the insula was always abnormal; (3) neocortical temporal lobe damage was usually unilateral or asymmetric. It never occurred in isolation, and was invariably associated with more medial pathological changes; (4) anterior and inferior temporal lobe gyri were damaged more often and more severely than posterior and superior temporal lobe gyri; (5) pronounced abnormality was often present in the substantia innominata (region of the basal forebrain/anterior perforated substance); (6) there was evidence of significant abnormality in the fornix; (7) there was evidence of damage to the mammillary bodies; (8) thalamic nuclei were affected in around 50% of cases, with damage usually unilateral; (9) frontal lobe damage was present in a few patients, and affected medial areas more than dorsolateral areas; (10) there was some involvement of the striatum, although this was usually unilateral and mild; (11) there was usually limited involvement of the cingulate gyrus and of the parietal and occipital lobes; (12) the cerebellum and brain stem were never damaged. Lesion covariance analysis indicated a close relation between the presence of abnormalities in temporal lobe and limbic-diencephalic regions. Unlike severe head injury, lesions in the temporal pole were not associated with the presence of lesions in the orbitofrontal cortex. Long term neuropsychological impairments were characterised by a dense amnesia in 60% of cases, and a less serve but noticeable anterograde memory impairment in the others. Naming and problem solving deficits were found in a small number of cases. Only two patients were able to return to open employment. Severity of amnesia showed a significant relation with severity of damage to medical limbic system structures such as the hippocampus, with bilateral damage being particularly important. By contrast, there was a minimal relation between memory loss and severity of damage to the thalamus, to lateral temporal lobe areas, or to the frontal lobes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertyn L. E. Magnetic resonance imaging in herpes simplex encephalitis. Australas Radiol. 1990 May;34(2):117–121. doi: 10.1111/j.1440-1673.1990.tb02825.x. [DOI] [PubMed] [Google Scholar]
- Cermak L. S. The encoding capacity of a patient with amnesia due to encephalitis. Neuropsychologia. 1976;14(3):311–326. doi: 10.1016/0028-3932(76)90025-7. [DOI] [PubMed] [Google Scholar]
- Coffey C. E., Wilkinson W. E., Weiner R. D., Parashos I. A., Djang W. T., Webb M. C., Figiel G. S., Spritzer C. E. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch Gen Psychiatry. 1993 Jan;50(1):7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Eslinger P. J., Damasio H., Van Hoesen G. W., Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol. 1985 Mar;42(3):252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Demaerel P., Wilms G., Robberecht W., Johannik K., Van Hecke P., Carton H., Baert A. L. MRI of herpes simplex encephalitis. Neuroradiology. 1992;34(6):490–493. doi: 10.1007/BF00598957. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C., Derouesne C., Signoret J. L., Gray F., Escourolle R., Castaigne P. Bilateral and limited amygdalohippocampal lesions causing a pure amnesic syndrome. Ann Neurol. 1985 Sep;18(3):314–319. doi: 10.1002/ana.410180307. [DOI] [PubMed] [Google Scholar]
- Hierons R., Janota I., Corsellis J. A. The late effects of necrotizing encephalitis of the temporal lobes and limbic areas: a clinico-pathological study of 10 cases. Psychol Med. 1978 Feb;8(1):21–42. doi: 10.1017/s0033291700006607. [DOI] [PubMed] [Google Scholar]
- Irle E., Wowra B., Kunert H. J., Hampl J., Kunze S. Memory disturbances following anterior communicating artery rupture. Ann Neurol. 1992 May;31(5):473–480. doi: 10.1002/ana.410310503. [DOI] [PubMed] [Google Scholar]
- Kritchevsky M., Squire L. R. Permanent global amnesia with unknown etiology. Neurology. 1993 Feb;43(2):326–332. doi: 10.1212/wnl.43.2.326. [DOI] [PubMed] [Google Scholar]
- Lacomis D., Khoshbin S., Schick R. M. MR imaging of paraneoplastic limbic encephalitis. J Comput Assist Tomogr. 1990 Jan-Feb;14(1):115–117. doi: 10.1097/00004728-199001000-00021. [DOI] [PubMed] [Google Scholar]
- Laurent B., Allegri R. F., Michel D., Trillet M., Naegele-Faure B., Foyatier N., Pellat J. Encéphalites herpétiques à prédominance unilatérale. Etude neuropsychologique au long cours de 9 cas. Rev Neurol (Paris) 1990;146(11):671–681. [PubMed] [Google Scholar]
- Lee A. W., Cheng L. O., Ng S. H., Tse V. K., O S. K., Au G. K., Poon Y. F. Magnetic resonance imaging in the clinical diagnosis of late temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Clin Radiol. 1990 Jul;42(1):24–31. doi: 10.1016/s0009-9260(05)81617-4. [DOI] [PubMed] [Google Scholar]
- Lencz T., McCarthy G., Bronen R. A., Scott T. M., Inserni J. A., Sass K. J., Novelly R. A., Kim J. H., Spencer D. D. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992 Jun;31(6):629–637. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- Nelson H. E. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976 Dec;12(4):313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. M., Gelb A., Girvin J. P., Hachinski V. C. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992 Sep;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Penfield W., Mathieson G. Memory. Autopsy findings and comments on the role of hippocampus in experiential recall. Arch Neurol. 1974 Sep;31(3):145–154. doi: 10.1001/archneur.1974.00490390027001. [DOI] [PubMed] [Google Scholar]
- Pietrini V., Nertempi P., Vaglia A., Revello M. G., Pinna V., Ferro-Milone F. Recovery from herpes simplex encephalitis: selective impairment of specific semantic categories with neuroradiological correlation. J Neurol Neurosurg Psychiatry. 1988 Oct;51(10):1284–1293. doi: 10.1136/jnnp.51.10.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidbauer M., Podreka I., Wimberger D., Oder W., Koch G., Wenger S., Goldenberg G., Asenbaum S., Deecke L. SPECT and MR imaging in herpes simplex encephalitis. J Comput Assist Tomogr. 1991 Sep-Oct;15(5):811–815. doi: 10.1097/00004728-199109000-00016. [DOI] [PubMed] [Google Scholar]
- Shenton M. E., Kikinis R., Jolesz F. A., Pollak S. D., LeMay M., Wible C. G., Hokama H., Martin J., Metcalf D., Coleman M. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992 Aug 27;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Warrington E. K., Duchen L. W. A re-appraisal of a case of persistent global amnesia following right temporal lobectomy: a clinico-pathological study. Neuropsychologia. 1992 May;30(5):437–450. doi: 10.1016/0028-3932(92)90091-y. [DOI] [PubMed] [Google Scholar]
- Warrington E. K., McCarthy R. A. The fractionation of retrograde amnesia. Brain Cogn. 1988 Apr;7(2):184–200. doi: 10.1016/0278-2626(88)90029-2. [DOI] [PubMed] [Google Scholar]
- Warrington E. K., Shallice T. Category specific semantic impairments. Brain. 1984 Sep;107(Pt 3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Weiller C., Chollet F., Friston K. J., Wise R. J., Frackowiak R. S. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992 May;31(5):463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Hadley D. M., Wiedmann K. D., Teasdale G. M. Intercorrelation of lesions detected by magnetic resonance imaging after closed head injury. Brain Inj. 1992 Sep-Oct;6(5):391–399. doi: 10.3109/02699059209008135. [DOI] [PubMed] [Google Scholar]
- Woods B. T., Schoene W., Kneisley L. Are hippocampal lesions sufficient to cause lasting amnesia? J Neurol Neurosurg Psychiatry. 1982 Mar;45(3):243–247. doi: 10.1136/jnnp.45.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986 Oct;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]