Abstract
Circular RNAs (circRNAs) are a class of single-stranded RNAs with covalently closed structures. Owing to their not having 3' or 5' ends, circRNAs are highly durable and insusceptible to exonuclease-mediated degradation. Moreover, some circRNAs with certain structures are translatable, making them novel vaccines. Vaccines are efficient tools for immunotherapy, such as for the prevention of infectious diseases and cancer treatment. The immune system is activated during immunotherapy to fight against abnormal allies or invaders. CircRNA vaccines represent a potential new avenue in the vaccine era. Recently, several circRNA vaccines have been synthesized and tested in vitro and in vivo. Our review briefly introduces the current understanding of the biology and function of translatable circRNAs, molecular biology, synthetic methods, delivery of circRNA, and current circRNA vaccines. We also discussed the challenges and future directions in the field by summarizing the developments in circRNA vaccines in the past few years.
Key words: circular RNA, vaccine, drug delivery, immunotherapy
Introduction
Vaccines are essential for the maintenance of human health. They are the most efficient tools for infectious disease prevention and among the most promising methods for fighting cancer. The vaccination era began in the late 18th century when Edward Jenner invented the first vaccine to induce preventive immunity in the human body to protect against deadly smallpox.[1] Since then, vaccine strategies have evolved from live pathogen inactivation or attenuation techniques to the construction of biologically modified proteins or peptide antigens and eventually to the use of nucleic acids to induce in vivo antigen-encoding.[2,3] Although advancements in conventional vaccine approaches have had success in controlling infectious diseases and cancer therapy, challenges remain, and breakthroughs in vaccine technologies are necessary.[4] For example, mRNA vaccines, such as BNT162B2[5,6] and Chadox1[7] have reduced the hospitalization rates of patients infected with the SARS-Cov-2 alpha-variant, but their effectiveness in delta-variant patients is considerably lower than that.[8, 9, 10] Moreover, the protein-encoding functions of circRNAs were demonstrated, and a new type of vaccine using circRNAs as a platform was developed and proved efficient in vitro and in vivo. These single-stranded RNAs are perfect vaccine carriers because they are highly durable and invulnerable to exonuclease owing to the inaccessibility of free 5’ or 3’ ends.[11] Moreover, they have several advantages that facilitate their use as cost-effective vaccines.
Overview of circular RNA
Structure and biogenesis
Circular RNA is a prominent feature of the transcriptomes of many metazoans, including those in humans.[12, 13, 14, 15] Once thought to be meaningless by-products of splicing errors,[16] circRNAs are expressed in cell-specific, tissue-specific, and developmental stage-specific patterns.[17, 18, 19] Known as a type of covalently closed single-stranded RNA without accessible 5’ or 3’ends, circRNAs are usually produced by classical linear splicing and RNA back-splicing.[20, 21, 22] Some circRNAs originate from tRNA splicing reactions.[23] For example, tric31905 is a circular RNA transcript derived from the fruit fly tRNA gene CR31905[23] and the circular tRNATrp intron is a highly stable circRNA in Haloferax volcanii.[24] Linear splicing generally forms intronic circRNAs, upon which an intron lariat that can avoid debranching is created when an upstream donor splice site is ligated to a downstream acceptor splice site.[20] Back-splicing is a process through which a 3′, 5′-phosphodiester bond is formed between the 3′-end of an exon and the 5′-end of either its or an upstream exon, creating a closed structure with a back-splicing junction site.[12,25] Most circRNAs are exon-containing and are derived from the back-splicing of precursor messenger RNA exons in a spliceosome-dependent manner. However, a small portion of intronic circRNAs originates from the processing of cellular non-coding sequences.[26] For example, circRNAs can be detected as processing intermediates during rRNA maturation in archaea.[24] circRNA biogenesis is modulated by various distinct factors.[17] Studies have revealed that back-splicing requires spliceosomal machinery as a catalyst and is regulated by both RNA-binding proteins and intronic complementary sequences.[27, 28, 29] However, additional regulatory factors that influence circRNA biogenesis require further research.
Function
circRNAs participate in various biological processes, such as cellular activity modulation, gene and protein sponging, cell proliferation, tumor progression, and immune regulation.[30, 31, 32, 33, 34, 35, 36, 37] For decades, circRNAs have been considered untranslatable because they do not have 5’caps,[38,39] and cap-independent translation was discovered to allow circRNA translation;[40] further investigations have provided evidence for their ubiquity.[41] Based on the previous study, Kozak consensus sequences, m6A-modification, and internal ribosome entry sites (IRES) can initiate protein translation from circRNA without 5′ caps.[20] The traits of circRNAs associated with their coding capability are as follows: (1) the presence of IRES in open reading frames (ORFs), N6-methyladenosine (m6A) RNA modification, or Kozak consensus sequences [42]; (2) ribosome association by ribosome profiling; and (3) translated peptides from back-splicing junction sites.[43] According to the literature, circRNAs with nuclear localization are virtually untranslated, and hundreds of circRNAs localized in the cytoplasm are translatable.[43,44] CircRNAs could be incorporated into the 40S subunit of eukaryotic ribosomes via IRES, which are upstream of the circRNA start codon (first identified in picornavirus mRNAs), and are thereby translated by ribosomes.[45] Evidence has shown that either the complementary regions of 18S rRNA or a structured RNA element in circRNAs can facilitate circRNA translation in an IRES-dependent manner.[46] Moreover, by recruiting the m6A reader YTHDF3 and translation initiation factor eIF4G2, m6A modifications can initiate circRNA translation.[47,48] Proteins translated from circRNAs are involved in various physiological functions, including the regulation of cell proliferation, differentiation, migration, and myogenesis.[49, 50, 51, 52, 53] The protein-coding ability of circRNAs enables their potential use as cancer vaccines.
Characteristics that enable circRNA’s use as vaccines
circRNAs have unique characteristics that enable their use as vaccines. Because circRNAs have a covalently closed structure and no termini, circRNAs are immune to degradation mediated by exonucleases, conserving a higher stability than their linear mRNA isoforms. [11,25,54,55] In mammalian cells, the intermediate half-life of circRNAs is at least 2.5 times more than that of their linear counterparts.[56] The safe antigen production of circRNA-based vaccines can be ensured by circRNAs’ ability to be expressed endogenously.[41,57–59] circRNAs such as circZNF609,[53] circMbl,[60] and circSfl [61] are associated with polyribosomes and can generate functional proteins that play a role in cell biology.[49,51,62] Without integrating into the genome, circRNA-based vaccines can be translated into antigens in the cytoplasm. In addition, another study demonstrated that synthetic circRNAs derived from foreign introns triggered effective immune responses and inhibited infection via the RNA pattern recognition receptor retinoic-acid-inducible gene-I-dependent pathway.[63] These findings indicate that circRNAs can induce innate immune responses and implies that their immunogenicity could be used in creating circRNA self-adjuvant vaccines.[63] Although literature has widely accepted that the translation efficiency of circRNAs in a CAP-independent manner is relatively lower than that of linear RNAs in a CAP-dependent manner, a recent study proposed a different view: circRNAs have high translation efficiency owing to their covalently closed topology. This unique structure allows ribosomes to pass over the length of circRNAs even after reaching a stop codon, which makes it easier to re-initiate translation.[64] In addition, studies have shown that the rolling circle translation of circRNAs is enabled by their covalently closed topology.[40,42,65] Moreover, as recent studies have demonstrated,[66,67] engineering circRNAs can enhance their translation efficiency and lead to more stable protein production than that of their linear counterparts.
Because of inherent stability, immunogenicity, achievable high translation efficiency through engineering, and an inevitable need for nucleotide modifications (which are required for mRNA production via the in vitro transcription [IVT] pathway for the improvement of mRNA stability without increasing the likelihood of unwanted immunogenicity[68]), we posit that circRNAs have the better potency to be developed into vaccines than mRNA.
Molecular biology of circRNA vaccine
Structure of vaccine
The circRNA vaccine is a subtype of the nucleic acid vaccine that can be delivered with or without a carrier; thus, the circRNA can be administered directly in a naked form or encapsulated in delivery carriers, such as lipid nanoparticles (LNPs). Modified circRNAs generally comprise coding regions for vaccine antigens, untranslated regions, promoters, IRES, permuted intron-exon (PIE) systems, RNA spacers, and homology arms. IRES are internal ribosome entry sites placed before the coding sequences to initiate translation. Homology arms and rationally designed spacers are constructed to increase circularization efficiency.[69]
Mechanisms of circRNA vaccine-mediated immunotherapy
CircRNA vaccines are promising candidates for disease-related immunotherapies, such as the prevention of infectious diseases and cancer treatment. CircRNA vaccines can generate corresponding proteins via translation after injection. These heterologous gene expression products can directly affect immune cells. They induce and reinforce innate and adaptive immunity by stimulating the activation and proliferation of immune cells, reinforcing the host’s ability to fight viruses and tumors. For example, Yang et al. reported that direct administration of a circular mRNA (cmRNA) mixture that encodes four cytokines into tumors can lead to T cell activation (both CD4+ and CD 8+ T cells) and promote immune cell penetration into the tumor, exerting a strong antitumor effect.[70]
There is evidence that the activation of immunostimulatory RNA receptors in cells can intrinsically induce immunogenic circRNAs.[63] Owing to their immunogenicity, circRNAs can serve as self-adjuvanted vaccinations. For example, in studies led by Qu L and colleagues, the CircRNARBD vaccine triggered distinct Th1-skewed immune responses and brought about neutralizing antibody elicitation in a large proportion of patients,[71] suggesting activated innate immune responses.
Advantages of circRNA vaccine
The most commonly used vaccines are based on pathogens, DNA, and proteins/peptides, each of which has distinct features and limitations. For example, mRNA-based vaccines have short half-lives and are susceptible to exonuclease digestion. Therefore, they require a strictly controlled sterile environment free of ribonucleases during the entire production procedure and a low-temperature cold chain for storage and distribution.[72] DNA-based vaccines have the potential for genome integration.[73]
Owing to their unique properties, circRNA vaccines are better than conventional vaccines. First, because circRNAs can produce more significant amounts of proteins for a longer duration than linear RNAs,[69] circRNA vaccines probably have high translation efficiency. The translated products would have prolonged expression. Second, the literature synthesized circRNAs that, even in modest quantities, expressed particular antigens highly immunogenic for DC presentation.[74] This feature results in circRNAs having potential for use in highly efficient vaccines. Third, circRNA-LNPs exhibited greater thermostability than linear mRNA-LNP vaccines. The literature reported that circular RNA vaccines encapsulated in LNPs were conserved well for no less than 4 weeks at 4°C and approximately 30 d at room temperature.[20]
However, the limitations and disadvantages of the circRNA vaccines remain unclear. For example, circRNA immunogenicity can be a double-edged sword. Although the immunogenicity of circRNAs enables highly efficient circRNA vaccines, whether this characteristic would inhibit vaccine development remains unclear.[75] Moreover, the safety concerns of circRNA vaccines require further research. In addition, the techniques for generating circRNA vaccines are immature for large-scale vaccine production. Thus, further investigations are necessary before circRNA vaccines can be used in clinical trials.
Approaches to synthesize circRNA vaccine
Generally, circRNAs are produced by the synthesis of one or more precursor linear RNAs, followed by RNA circularization to generate a covalently closed loop, which is often mediated by chemical or enzymatic ligation methods.[76]
Designing the constructs/backbone of circRNA and linear RNA
A significant challenge in the synthesis of circRNAs is their inability to progress through the standard cap-dependent procedure used to initiate translation. Several strategies have been developed to overcome this limitation. Two main mechanisms allow for endogenous circRNA translation in a cap-independent manner: m6A alteration of the region located close upstream of the start codon and a virally originated IRES upstream of the circular sequence containing the ORF. m6A modification refers to the methylation of N6 in the nitrogenous base adenine,[77] which is the most pervasive modification in eukaryotes.[78] IRES, a structured RNA element located upstream of the start codon, can initiate translation without the presence of a 5′ cap by recruiting eukaryotic ribosomes.[65] The m6A modification is preferable to viral IRESs when considering construct size.[79] However, the two strategies can be combined. For example, m6A increases the effectiveness of circZNF609 translation mediated by IRES. [53,80] Moreover, a recent study improved circRNA translation. According to Chen et al., the optimization of these five elements—5’ and 3’ UTRs, vector topology, synthetic aptamers, and IRESs—can not only lead to an increase in protein yields of circRNA by several hundred folds but also enable powerful, long-lasting protein production in vivo.[66]
Synthesis of precursor linear RNA
There are two methods for synthesizing the precursor linear RNAs for circRNA synthesis: chemical and enzymatic.[81,82] Techniques for RNA chemical synthesis depend on synthesizer machines. Phosphoramidites, derivatives of nucleotide triphosphates, are used in the procedure as fundamental components of linear oligonucleotides.[79] Phage RNA polymerases are used in enzymatic strategies for IVT. An IVT reaction is usually conducted using the following main components: a double-stranded DNA template, ribonucleotide triphosphates, and DNA-dependent RNA polymerase.[79]
RNA circularization in vitro
Generally, exogenous RNA circularization can be achieved using three strategies: chemically, using cyanogen bromide or a similar condensing agent; enzymatically, using DNA or RNA ligases; or ribozymatically, using self-splicing introns.[69]
Chemical strategies
The most commonly used approach is applying 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide or the condensation reagent cyanogen bromide to activate RNA circularization.[83] Additional chemical methods rely on replacing native phosphate and hydroxyl groups of linear RNA with other functional groups. These alternative reactive groups help form bonds with excellent selectivity and efficacy, increasing the purity and quantities of circularized RNA.[84]
Enzymatic strategies
Three types of polynucleotide ligases are commonly used in the enzymatic ligation of synthetic oligonucleotides: T4 RNA ligase 1, T4 DNA ligase, and T4 RNA ligase 2. They are encoded in the genome of the bacteriophage T4 and can ligate nicks in single-and/or double-stranded RNA constructs. [85,86] They assist in the circularization process by promoting the ATP-consuming synthesis of a phosphodiester bond between the 3′-hydroxyl (acceptor) and 5′-phosphate (donor) end groups in RNA or DNA.[87]
Ribozymatic strategies
Ribozymatic strategies are the most commonly used methods for RNA circularization, particularly when the production of large circular RNAs is required.[88] Group I and group II introns play a role in circRNA production in vitro.[89, 90, 91] However, the use of group I introns is more common than that of group II. In 1992, Puttaraju and Been reported the first use of the group I intron system to synthesize circRNAs from a linear precursor containing introns. The Anabaena pre-tRNALeu gene was used to form a PIE construct, in which the 5′half of the group I tRNA intron was moved to the tail of the tRNA exon, the remaining 3′half of the intron was placed at the head of the same exon, and circRNAs were formed through exon ligation.[92] The in vitro circularization of RNAs can be achieved more efficiently than by using the aforementioned method by using group II introns and does not require native exons.[89] In this pathway, the circularized exon and the excised group II intron are joined together by 3′-5′ and 2′-5′ phosphodiester linkages separately. However, the exact mechanism underlying this process remains unclear.[93]
Delivery of circRNA vaccine
The goal of a vaccine is not only to elicit an initial response to specific antigens but also to promote an enduring response from the body’s adaptive immune system.[94] By exposing the immune system to a particular antigen (or antigens), a vaccine can generate long-lived protective immunity in the form of memory T cells, memory B cells, and antibody-producing plasma cells.[95] Appropriate vaccine delivery is vital to guarantee successful vaccination.
CircRNAs function by producing specific antigens in the host’s cytoplasm. However, they are too large to diffuse freely across cell membranes. CircRNAs might benefit from RNA delivery mechanisms already present, and their distinct structural features might help with formulation and distribution. The terminus-free structure of circRNAs enables resistance to exonuclease degradation, making them suitable for carrier-free administration.[96] For example, naked cmRNA dissolved in PBS was directly injected into the tumor tissues of four types of tumor models, resulting in the detection of its specific expression product.[70] The literature demonstrated that conventional physical techniques such as gene guns, electroporation, and microneedles improved the efficacy of naked mRNA antigen presentation.[97] These results imply that the same methods can be used to facilitate the administration of naked circRNA vaccines. CircRNAs can be delivered to carriers such as nanoparticles. Among all mRNA delivery vehicles, the lipid-based nanoparticle delivery system is the most efficient and occupies the leading status.[98] Its advantages include biocompatibility, ease of formation, modularity, and substantial payload capacity.[99] Moreover, the relatively compact structure of circRNAs may contribute to their high loading capacity into RNA delivery carriers such as viral carriers or nanoparticles.[20] In improving their efficiency, circRNAs are often administered via nanocarriers. For instance, purified hEpo circRNAs encased in LNPs demonstrated robust expression when injected into 293 cells, demonstrating the potency of in vivo circRNA delivery via lipid nanoparticles.[100] Although researchers have managed to deliver functional circRNAs in naked and carrier-facilitated forms, the use of RNA delivery carriers, especially nanoparticles, is a more commonly used method for vaccine delivery.
Immune reactivity after vaccine injection is a key factor affecting vaccine function. The immune response begins as soon as the circRNA reaches the cytoplasm. In this study, we used an LNP-encapsulated circRNA vaccine. After the injection of a circRNA vaccine, circRNA-LNPs are taken up by cells through fusion with the endosomal membrane. Next, circRNAs are translated into proteins by ribosomes after they enter the cytosol. These antigens can trigger immune responses as follows [101]: (1) The proteasome complex breaks down intracellular antigens into smaller molecules, which are then displayed on the cell surface of CD8+ T cells via attachment to MHC I (MHC I is present on the surface of almost all nucleated cells). T lymphocytes are activated and prepare to provoke a cellular immune response when they recognize and attach to epitope-bound MHC I receptors. Once activated, cytotoxic T cells excrete cytokines such as TNFα and IFN-γ, as well as cytolytic molecules, such as perforin and granzyme. Proteases can enter targeted cells through the cell membrane pores created by these cytotoxic chemicals, initiate viral protein degradation, and bolster cellular apoptosis. (2) Secreted antigens can be absorbed by antigen-presenting cells (APCs), broken down inside endosomes, and displayed on the cell surface by MHC class II proteins. When the APC is a B lymphocyte, it can develop into a plasma cell and release IgM antibodies. When a CD4+ T cell interacts with the APC, the CD4+ T cell becomes activated; facilitates the activation of phagocytes; and advocates B cell proliferation, as well as somatic hypermutation. This procedure generates antibody variants with high antigen affinities to yield highly efficient and specific immune responses. These B cells can either be retained as memory B cells or distributed in the bloodstream as plasma cells that secrete IgG antibodies. The APC can travel to the body’s lymph nodes, participate in T cell-dependent B cell maturation, and aid in the development of a more potent and persistent humoral immune response against the vaccine antigen [99, 102] (Figure 1).
Figure 1.
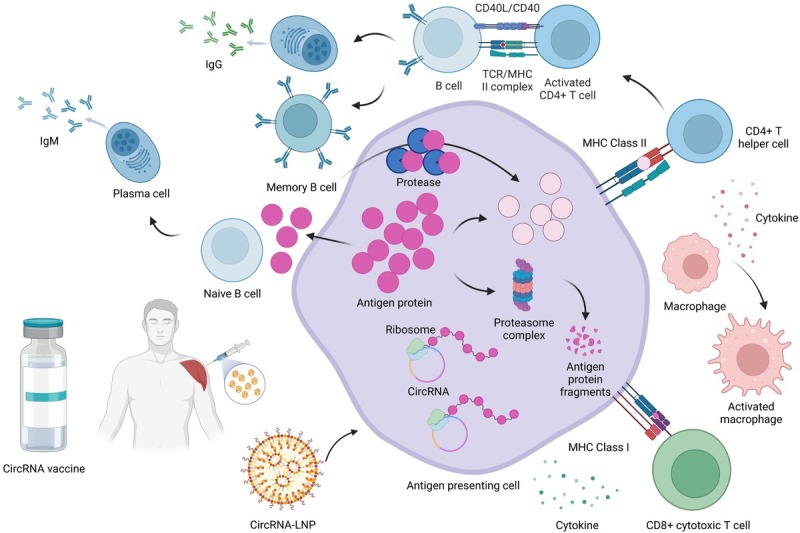
CircRNA in vivo delivery and immunity activation. A circRNA vaccine is administered via the intramuscular route into the deltoid. circRNA-LNPs are taken up by an APC (antigen-presenting cell) through fusion with the endosomal membrane. CircRNAs combine with ribosomes and are translated into antigen proteins. The intracellular antigen is broken down into smaller fragments by the proteasome complex, and the fragments are displayed on the cell surface to cytotoxic T cells (CD8+ T cells) via attachment to MHC I (MHC I is expressed on the surface of nucleated bodily cells). Cytotoxic T cells (CD8+ T cells) become activated through recognition and binding to the epitope-bound MHC I receptor, secreting cytokines and cytolytic molecules and thus initiating the cellular immune response. Translated antigen proteins can be secreted outside the cell. These secreted antigens can activate a B cell through BCR (B cell receptor) and make it transform into a plasma cell that secretes IgM antibodies. They can also be taken up by APCs, degraded inside endosomes, and presented on the cell surface by MHC class II proteins. When a CD4+ T cell interacts with the APC, it becomes activated and facilitates the activation of macrophages and somatic hypermutation within B cells. These B cells can either be stored as memory cells or disseminate into the blood as plasma cells secreting IgG antibodies.
Current circRNA vaccines
Viral infectious disease
CircRNARBD: SARS-CoV-2 receptor-binding domain (RBD) encoding circRNA vaccine
In this study, Qu L and colleagues synthesized a circRNA vaccine that encoded SARS-CoV-2 RBD antigens by using both ribozymatic and enzymatic strategies in RNA circularization (described by Wesselhoeft et al.) and encapsulated the circRNAs with LNPs. circRNA-encoded RBD antigens are also functional. Mice (female BALB/c mice aged 6–8 weeks) and rhesus macaques (male, aged 2–4 years) effectively elicited sufficient neutralizing antibodies and potent specific T cell responses against SARS-CoV-2. Moreover, compared with its mRNA counterpart, this circRNA vaccine induced larger quantities of immunogens that were more durable and evoked a greater median proportion of protective antibodies against SARS-CoV-2 and their emerging variants, such as Omicron and Delta and Th1-skewed immune responses. In addition, the study proved that the delta-specific vaccine circRNARBD-Delta could provide cross-protection against all other variants, including Omicron, and could serve as an efficient booster after two doses of the initial SARS-CoV-2 vaccination. CircRNAs that encode SARS-CoV-2-specific neutralizing nanobodies, as well as hACE2 decoys, were also tested for their therapeutic potential and were effective in neutralizing SARS-CoV-2 pseudovirus. Furthermore, in vaccinated nonhuman primates, the circRNA vaccine did not cause clinical signs of sickness or exacerbated pathology, which may be proof of its safety.[71]
VFLIP-X: a SARS-CoV-2 circRNA vaccine
In the present study, VFLIP-X, a circRNA vaccine targeting SARS-CoV-2, was developed to test its potential as a new-generation COVID-19 vaccine. For the in vivo experiments to test the validity of this vaccine, 7-week-old female BALB/C mice were used. VFLIP is a spike protein engineered using one flexible S1/S2 linker, five proline replacements in the S2 subunit, and two cysteine substitutions to induce intermolecular disulfide bond formation. VFLIP-X contains six reasonably replaced amino acids rationally chosen based on the co-mutation (D614G) discovered in all SARS-CoV-2 variants and five mutations (E484K, K417N, L452R, N501Y, and T478K) co-identified in multiple variants of concern and variants of interest. The circRNAs were produced by T7 RNA polymerase-based in vitro transcription and encapsulated in LNPs. According to the research, VFLIP-X imparts neutralization against SARS-CoV-2, stimulates the generation of cross-neutralizing antibodies against SARS-CoV-2 variants, and activates humoral and cellular immune responses against B. 1.1.529 variant. This study identified that a SARS-CoV-2 circRNA vaccine encoding a relatively stable VFLIP-X spike immunogen would be suitable as a next-generation COVID-19 vaccine guarding against existing and developing SARS-CoV-2 variants.[103]
Tumor immune therapy
Cytokine-encoding circular mRNA for cancer therapy
An innovative form of circular mRNA, named cmRNA, was developed in the study. It is produced using the PIE system, which utilizes a novel spacer1 and E29 IRES to guide protein translation and is encapsulated in LNPs. This type of circular mRNA is more resilient than linear mRNA and can mediate a larger amount of protein production than linear mRNA can. Tumor cells and mouse models were used for the in vitro and in vivo experiments, respectively. The in vitro and in vivo experiments confirmed its ability to efficiently express various proteins, suggesting its use as a universal vector for protein expression. Intratumoral injection of a cmRNA compound encoding four cytokines induced a significant tumor-repressive effect. It activates immune cells, especially T cells, and collaborates with anti-PD-1 antibodies to promote total immune cell penetration into the tumor, exerting a strong antitumor effect. These results indicate that this novel type of naked cmRNA has the potential to serve as an RNA platform for the development of various intratumoral therapies.[70]
CircRNAOVA-luc-LNP vaccine
A circRNAOVA-luc-LNP (OVA[257-264]-luciferase-coding circRNA) vaccine was constructed using the PIE system and encapsulated in LNPs. Three mouse tumor models were established to evaluate the efficacy of the vaccine. circRNAs demonstrate better stability and trigger longer-lasting protein expression. circRNA-LNPs elicited a potent innate immunological response and a remarkable antigen-specific response. This circRNA-LNP vaccine has shown great efficacy in suppressing the advancement of immune-exclusive tumors, inducing complete tumor regression in immunological desert tumors, and preventing cancer cell metastasis. In addition, circRNA-LNPs can collaborate with adoptive cell transfer therapy and completely repress the progression of late-stage immune-exclusive tumors by reinforcing the persistence of TCR-T cells, demonstrating the potential of RNA vaccines as tumor therapeutics.[104]
As shown in Table 1, the characteristics of the circRNA vaccine, including its high stability, efficacy in protein expression, stable and durable expression products, and ability to initiate immune responses, are why it is an attractive alternative to the aforementioned vaccines. Additionally, circRNA vaccines are superior to mRNA vaccines. Moreover, its manufacturing procedures and preservation conditions are simple and economical, making it more suitable for mass production and wide applications.
Table 1.
Comparison of the emerging circRNA vaccines
| Vaccine | Target immunogen | RNA circularization strategy | Delivery method | Structure to initiate translation |
|---|---|---|---|---|
| circRNARBD | SARS-CoV-2 RBD (receptor- binding domain) antigen | Enzymatic and ribozymatic (T4 RNA (group ligase) I intron autocatalysis strategy) | LNP | CVB3 IRES |
| VFLIP-X | SARS-VFLIP-CoV-X 2 spike protein | Enzymatic (T4 RNA ligase) | LNP | CVB3 IRES |
| Cytokine-encoding circular mRNA | 4 cytokines (active IL-15, IL- 12sc, GM-CSF, IFN-a 2b) | Ribozymatic (group I intron autocatalysis strategy) | LNP | E29 IRES |
| circRNAOVA-luc-LNP | OVA (257–264)-luciferase | Ribozymatic autocatalysis (group strategy) I intron | LNP | CVB3 IRES |
Conclusions
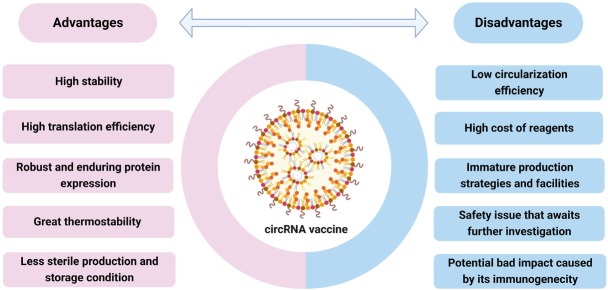
Owing to the many advantages of circRNA, for example, high stability and achievable high translation efficiency, we used it as a vaccine. However, additional studies and improvements are required before circRNA vaccination becomes widely available. The literature has shown that circRNA vaccines do not lead to clinical signs of sickness, and further investigation is required to determine the safety of circRNA vaccines. Moreover, the impact of circRNA immunogenicity on vaccine development, which may act as an inhibitor of vaccine development, remains unclear. The classical methods described by Wesselhoeft et al. have been adopted and successfully used in the literature aiming to generate circRNA vaccines. However, limitations persist in the current circRNA synthesis techniques, such as low circularization productivity and the high expense of reagents such as enzymes. The stage of the current production strategies and facilities is too immature to produce massive quantities, which is an important factor restricting the application of circRNA vaccines (Figure 2). With future technological advances, we envision a broad application of circRNA vaccines in clinical trials to prevent infectious diseases and suppress tumor malignancies.
Figure 2.
Advantages and disadvantages of circRNA vaccine.
Funding Statement
This research was supported by the China Postdoctoral Science Foundation (2022M720178, Shaoquan Zheng).
Footnotes
Author Contributions
Research design: Yutian Zou and Shaoquan Zheng; data collection: Jindong Xie and Fengxi Ye; data analysis: Jindong Xie, Fengxi Ye, and Xinpei Deng; manuscript preparation: Jindong Xie, Fengxi Ye, Xinpei Deng, Yuhui Tang, Jie-Ying Liang, Xufeng Huang, Yuying Sun, Hailin Tang, and Jinsong Lei; manuscript editing: Yutian Zou and Shaoquan Zheng. All authors confirm that they contributed to manuscript reviews and critical revision for important intellectual content and read and approved the final draft for submission. All authors agree to be accountable for the contents of the study. All the authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Contributor Information
Shaoquan Zheng, Email: zhengshq3@mail2.sysu.edu.cn.
Yutian Zou, Email: zouyt@sysucc.org.cn.
References
- 1.Meyer H, Ehmann R, Smith GL. Smallpox in the Post-Eradication Era. Viruses. 2020;12:138. doi: 10.3390/v12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis MJ. Recent Advances in Vaccine Technologies. Vet Clin North Am Small Anim Pract. 2018;48:231. doi: 10.1016/j.cvsm.2017.10.002. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011;9:889. doi: 10.1038/nrmicro2668. –. [DOI] [PubMed] [Google Scholar]
- 4.Soni D, Van Haren SD, Idoko OT, Evans JT, Diray-Arce J, Dowling DJ. Towards Precision Vaccines: Lessons From the Second International Precision Vaccines Conference. Front Immunol. 2020;11:590373. doi: 10.3389/fimmu.2020.590373. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603. doi: 10.1056/NEJMoa2034577. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S. Publisher Correction: Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2021;590(7844):E26. doi: 10.1038/s41586-020-03098-3.. et al. Feb. doi. Erratum for: Nature 2020;586:589-593. [DOI] [PubMed] [Google Scholar]
- 7.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK. Safety and efficacy of the ChAdOx1 nCoV- 19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99. doi: 10.1016/S0140-6736(20)32661-1. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, He Y, Jie Z. Delta Variant: Partially Sensitive To Vaccination, but Still Worth Global Attention. J Transl Int Med. 2022;10:227. doi: 10.2478/jtim-2022-0026. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646. doi: 10.1016/S0140-6736(21)00677-2. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S. Effectiveness of Covid- 19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585. doi: 10.1056/NEJMoa2108891. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453. doi: 10.1038/nbt.2890. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, Xu RH. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17:679. doi: 10.1038/nrg.2016.114. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y, Zou Y, Gao G, Zheng S, Wu S, Xie X. The biogenesis, function and clinical significance of circular RNAs in breast cancer. Cancer Biol Med. 2021;19:14. doi: 10.20892/j.issn.2095-3941.2020.0485. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD. Scrambled exons. Cell. 1991;64:607. doi: 10.1016/0092-8674(91)90244-s. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503. doi: 10.7150/thno.42174. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A, Jiang B, Song C, Zhong Q, Mo Y, Yang R. Isoliquiritigenin inhibits circ0030018 to suppress glioma tumorigenesis via the miR-1236/HER2 signaling pathway. MedComm. 2020;4:e282. doi: 10.1002/mco2.282. et al. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zhang Y, Zhou S, Dain L, Mei L, Zhu G. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022;348:84. doi: 10.1016/j.jconrel.2022.05.043. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Liu S, Zhang L, Issaian A, Hill RC, Espinosa S. A unified mechanism for intron and exon definition and back-splicing. Nature. 2019;573:375. doi: 10.1038/s41586-019-1523-6. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Wang Z, Zhang Z, Zhang W, Zhang M, Shen Z. Landscape of cell heterogeneity and evolutionary trajectory in ulcerative colitis-associated colon cancer revealed by single-cell RNA sequencing. Chin J Cancer Res. 2021;33:271. doi: 10.21147/j.issn.1000-9604.2021.02.13. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z, Filonov GS, Noto JJ, Schmidt CA, Hatkevich TL, Wen Y. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA. 2015;21:1554. doi: 10.1261/rna.052944.115. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danan M, Schwartz S, Edelheit S, Sorek R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012;40:3131. doi: 10.1093/nar/gkr1009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675. doi: 10.1038/s41576-019-0158-7. –. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Wilusz JE, Chen LL. Biogenesis and Regulatory Roles of Circular RNAs. Annu Rev Cell Dev Biol. 2022;38:263. doi: 10.1146/annurev-cellbio-120420-125117. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428. doi: 10.1016/j.molcel.2018.06.034. –. [DOI] [PubMed] [Google Scholar]
- 28.Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103. doi: 10.1016/j.celrep.2014.12.002. et al. –. [DOI] [PubMed] [Google Scholar]
- 29.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55. doi: 10.1016/j.molcel.2014.08.019. et al. –. [DOI] [PubMed] [Google Scholar]
- 30.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55. doi: 10.1016/j.molcel.2014.08.019. et al. –. [DOI] [PubMed] [Google Scholar]
- 31.Lei M, Zheng G, Ning Q, Zheng J, Dong D. Translation and functional roles of circular RNAs in human cancer. Mol Cancer. 2020;19:30. doi: 10.1186/s12943-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869. doi: 10.1016/j.cell.2018.12.021. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia P, Wang S, Ye B, Du Y, Li C, Xiong Z. A Circular RNA Protects Dormant Hematopoietic Stem Cells from DNA Sensor cGAS-Mediated Exhaustion. Immunity. 2018;48:688. doi: 10.1016/j.immuni.2018.03.016. et al. –. [DOI] [PubMed] [Google Scholar]
- 35.Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014;9:1966. doi: 10.1016/j.celrep.2014.10.062. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao MS, Ai Y, Wilusz JE. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020;30:226. doi: 10.1016/j.tcb.2019.12.004. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Deng X, Chen X, Chang Z, Lu Q, Tang A. Circular RNA KIF4A Promotes Liver Metastasis of Breast Cancer by Reprogramming Glucose Metabolism. J Oncol. 2022;2022:8035083. doi: 10.1155/2022/8035083. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333. doi: 10.1038/nature11928. et al. –. [DOI] [PubMed] [Google Scholar]
- 39.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perriman R, Ares M Jr. Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo. RNA. 1998;4:1047. doi: 10.1017/s135583829898061x. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L. Translation of CircRNAs. Mol Cell. 2017;66:9. doi: 10.1016/j.molcel.2017.02.021. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abe N, Matsumoto K, Nishihara M, Nakano Y, Shibata A, Maruyama H. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci Rep. 2015;5:16435. doi: 10.1038/srep16435. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CX, Chen LL. Circular RNAs: characterization, cellular roles, and applications. Cell. 2022;185:2016. doi: 10.1016/j.cell.2022.04.021. –. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792. doi: 10.1016/j.molcel.2013.08.017. et al. –. [DOI] [PubMed] [Google Scholar]
- 45.Rahmani-Kukia N, Abbasi A. New insights on circular RNAs and their potential applications as biomarkers, therapeutic agents, and preventive vaccines in viral infections: with a glance at SARS-CoV-2. Mol Ther Nucleic Acids. 2022;29:705. doi: 10.1016/j.omtn.2022.08.012. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CK, Cheng R, Demeter J, Chen J, Weingarten-Gabbay S, Jiang L. Structured elements drive extensive circular RNA translation. Mol Cell. 2021;81:4300. doi: 10.1016/j.molcel.2021.07.042. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626. doi: 10.1038/cr.2017.31. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang C, Xie Y, Yu T, Liu N, Wang Z, Woolsey RJ. m6A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020;30:211. doi: 10.1038/s41422-020-0279-8. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84. doi: 10.1186/s13059-019-1685-4. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J Natl Cancer Inst. 2018;110:304. doi: 10.1093/jnci/djx166. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475. doi: 10.1038/s41467-018-06862-2. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37:1805. doi: 10.1038/s41388-017-0019-9. et al. –. [DOI] [PubMed] [Google Scholar]
- 53.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol Cell. 2017;66:22. doi: 10.1016/j.molcel.2017.02.017. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju M, Kim D, Son G, Han J. Circular RNAs in and out of Cells: Therapeutic Usages of Circular RNAs. Mol Cells. 2023;46:33. doi: 10.14348/molcells.2023.2170. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer JW, Leung AK. CircRNAs: a regulator of cellular stress. Crit Rev Biochem Mol Biol. 2017;52:220. doi: 10.1080/10409238.2016.1276882. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370. doi: 10.1093/nar/gkv1367. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramer MC, Liang D, Tatomer DC, Gold B, March ZM, Cherry S. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev. 2015;29:2168. doi: 10.1101/gad.270421.115. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol Cell. 2017;67:214. doi: 10.1016/j.molcel.2017.05.023. et al. –. [DOI] [PubMed] [Google Scholar]
- 59.Wilusz JE. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip Rev RNA. 2018;9:e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L. Translation of CircRNAs. Mol Cell. 2017;66:9. doi: 10.1016/j.molcel.2017.02.021. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weigelt CM, Sehgal R, Tain LS, Cheng J, Eßer J, Pahl A. An Insulin-Sensitive Circular RNA that Regulates Lifespan in Drosophila. Mol Cell. 2020;79:268. doi: 10.1016/j.molcel.2020.06.011. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu C, Zhou N, Wang Z, Li G, Kou Y, Yu S. circGprc5a Promoted Bladder Oncogenesis and Metastasis through Gprc5a-Targeting Peptide. Mol Ther Nucleic Acids. 2018;13:633. doi: 10.1016/j.omtn.2018.10.008. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol Cell. 2017;67:228. doi: 10.1016/j.molcel.2017.05.022. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abe N, Hiroshima M, Maruyama H, Nakashima Y, Nakano Y, Matsuda A. Rolling circle amplification in a prokaryotic translation system using small circular RNA. Angew Chem Int Ed Engl. 2013;52:7004. doi: 10.1002/anie.201302044. et al. –. [DOI] [PubMed] [Google Scholar]
- 65.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415. doi: 10.1126/science.7536344. –. [DOI] [PubMed] [Google Scholar]
- 66.Chen R, Wang SK, Belk JA, Amaya L, Li Z, Cardenas A. Engineering circular RNA for enhanced protein production. Nat Biotechnol. 2023;41:262. doi: 10.1038/s41587-022-01393-0. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costello A, Lao NT, Barron N, Clynes M. Continuous translation of circularized mRNA improves recombinant protein titer. Metab Eng. 2019;52:284. doi: 10.1016/j.ymben.2019.01.002. –. [DOI] [PubMed] [Google Scholar]
- 68.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261. doi: 10.1038/nrd.2017.243. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Zhu J, Sun J, Chen Y, Du Y, Tan Y. Intratumoral delivered novel circular mRNA encoding cytokines for immune modulation and cancer therapy. Mol Ther Nucleic Acids. 2022;30:184. doi: 10.1016/j.omtn.2022.09.010. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu L, Yi Z, Shen Y, Lin L, Chen F, Xu Y. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. 2022;185:1728. doi: 10.1016/j.cell.2022.03.044. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics. 2020;12:102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hobernik D, Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018;19:3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szabó GT, Mahiny AJ, Vlatkovic I. COVID-19 mRNA vaccines: Platforms and current developments. Mol Ther. 2022;30:1850. doi: 10.1016/j.ymthe.2022.02.016. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ti D, Yan X, Wei J, Wu Z, Wang Y, Han W. Inducing immunogenic cell death in immuno-oncological therapies. Chin J Cancer Res. 2022;34:1. doi: 10.21147/j.issn.1000-9604.2022.01.01. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petkovic S, Müller S. Synthesis and Engineering of Circular RNAs. Methods Mol Biol. 2018;1724:167. doi: 10.1007/978-1-4939-7562-4_14. –. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Muisha MB, Zhang J, Sun Y, Li Z. Research Progress on N6-adenosylate Methylation RNA Modification in Heart Failure Remodeling. J Transl Int Med. 2023;10:340. doi: 10.2478/jtim-2022-0025. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635. doi: 10.1016/j.cell.2012.05.003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Obi P, Chen YG. The design and synthesis of circular RNAs. Methods. 2021;196:85. doi: 10.1016/j.ymeth.2021.02.020. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Timoteo G, Dattilo D, Centrón-Broco A, Colantoni A, Guarnacci M, Rossi F. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020;31:107641. doi: 10.1016/j.celrep.2020.107641. et al. [DOI] [PubMed] [Google Scholar]
- 81.Usman N, Cedergren R. Exploiting the chemical synthesis of RNA. Trends Biochem Sci. 1992;17:334. doi: 10.1016/0968-0004(92)90306-t. –. [DOI] [PubMed] [Google Scholar]
- 82.Rong M, He B, McAllister WT, Durbin RK. Promoter specificity determinants of T7 RNA polymerase. Proc Natl Acad Sci U S A. 1998;95:515. doi: 10.1073/pnas.95.2.515. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dolinnaya NG, Sokolova NI, Ashirbekova DT, Shabarova ZA. The use of BrCN for assembling modified DNA duplexes and DNA-RNA hybrids; comparison with water-soluble carbodiimide. Nucleic Acids Res. 1991;19:3067. doi: 10.1093/nar/19.11.3067. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Müller S, Appel B. In vitro circularization of RNA. RNA Biol. 2017;14:1018. doi: 10.1080/15476286.2016.1239009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller ES, Kutter E, Mosig G, Arisaka F, Kunisawa T, Rüger W. Bacteriophage T4 genome. Microbiol Mol Biol Rev. 2003;67:86. doi: 10.1128/MMBR.67.1.86-156.2003. - table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Remaut E, Tsao H, Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983;22:103. doi: 10.1016/0378-1119(83)90069-0. –. [DOI] [PubMed] [Google Scholar]
- 87.Petkovic S, Müller S. RNA circularization strategies in vivo and in vitro. Nucleic Acids Res. 2015;43:2454. doi: 10.1093/nar/gkv045. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Müller S, Appel B. In vitro circularization of RNA. RNA Biol. 2017;14:1018. doi: 10.1080/15476286.2016.1239009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mikheeva S, Hakim-Zargar M, Carlson D, Jarrell K. Use of an engineered ribozyme to produce a circular human exon. Nucleic Acids Res. 1997;25:5085. doi: 10.1093/nar/25.24.5085. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jarrell KA. Inverse splicing of a group II intron. Proc Natl Acad Sci U S A. 1993;90:8624. doi: 10.1073/pnas.90.18.8624. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puttaraju M, Been MD. Circular ribozymes generated in Escherichia coli using group I self-splicing permuted intron-exon sequences. J Biol Chem. 1996;271:26081. doi: 10.1074/jbc.271.42.26081. –. [DOI] [PubMed] [Google Scholar]
- 92.Puttaraju M, Been MD. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992;20:5357. doi: 10.1093/nar/20.20.5357. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray HL, Mikheeva S, Coljee VW, Turczyk BM, Donahue WF, Bar-Shalom A. Excision of group II introns as circles. Mol Cell. 2001;8:201. doi: 10.1016/s1097-2765(01)00300-8. et al. –. [DOI] [PubMed] [Google Scholar]
- 94.Orme IM, Henao-Tamayo MI. Trying to See the Forest through the Trees: Deciphering the Nature of Memory Immunity to Mycobacterium tuberculosis. Front Immunol. 2018;9:461. doi: 10.3389/fimmu.2018.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Irvine DJ, Aung A, Silva M. Controlling timing and location in vaccines. Adv Drug Deliv Rev. 2020;158:91. doi: 10.1016/j.addr.2020.06.019. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141. doi: 10.1261/rna.035667.112. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun X, Zeng L, Huang Y. Transcutaneous delivery of DNA/mRNA for cancer therapeutic vaccination. J Gene Med. 2019;21:e3089. doi: 10.1002/jgm.3089. [DOI] [PubMed] [Google Scholar]
- 98.Tam YY, Chen S, Cullis PR. Advances in Lipid Nanoparticles for siRNA Delivery. Pharmaceutics. 2013;5:498. doi: 10.3390/pharmaceutics5030498. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817. doi: 10.1038/s41573-021-00283-5. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol Cell. 2019;74:508. doi: 10.1016/j.molcel.2019.02.015. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartlett BL, Pellicane AJ, Tyring SK. Vaccine immunology. Dermatol Ther. 2009;22(2):104. doi: 10.1111/j.1529-8019.2009.01223.x. –. [DOI] [PubMed] [Google Scholar]
- 102.Hill A, Beitelshees M, Pfeifer BA. Vaccine Delivery and Immune Response Basics. Methods Mol Biol. 2021;2183:1. doi: 10.1007/978-1-0716-0795-4_1. –. [DOI] [PubMed] [Google Scholar]
- 103.Seephetdee C, Bhukhai K, Buasri N, Leelukkanaveera P, Lerdwattanasombat P, Manopwisedjaroen S. A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera. Antiviral Res. 2022;204:105370. doi: 10.1016/j.antiviral.2022.105370. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li H, Peng K, Yang K, Ma W, Qi S, Yu X. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics. 2022;12:6422. doi: 10.7150/thno.77350. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]