ABSTRACT
Invasive meningococcal disease (IMD) is a life-threatening disease caused by meningococcal serogroups A, B, C, W, X, and Y, of which B and W are most common in Argentina. The 4-component meningococcal serogroup B (4CMenB) vaccine contains three purified recombinant protein antigens (Neisseria adhesin A [NadA], factor H binding protein [fHbp], and Neisserial Heparin Binding Antigen [NHBA]) and outer membrane vesicles (OMV), which is derived from the New Zealand epidemic strain and contains Porin A 1.4. These antigens are present and conserved in strains that belong to other serogroups. In this study, we show that 10/11 (91%) meningococcal serogroup W (MenW) strains selected to be representative of MenW isolates that caused IMD in Argentina during 2010–2011 were killed in bactericidal assays by the sera of adolescents and infants who had been immunized with the 4CMenB vaccine. We also show that MenW strains that caused IMD in Argentina during 2018–2021 were genetically similar to the earlier strains, indicating that the 4CMenB vaccine would likely still provide protection against current MenW strains. These data highlight the potential of 4CMenB vaccination to protect adolescents and infants against MenW strains that are endemic in Argentina.
KEYWORDS: Meningococcal B, Meningococcal W, cross-protection, 4CMenB vaccine, vaccination, Argentina
Brief report
Invasive meningococcal disease (IMD), e.g. meningitis and septicemia, is a life-threatening disease.1 In Argentina, the incidence of meningococcal disease increased from approximately 0.46 per 100,000 people in 2010 to 0.70/100,000 people in 2013, but then declined to around 0.22 per 100,000 people in 2019.2 The incidence reduced further, to approximately 0.05 per 100,000 during 2020 and 2021, likely due to measures put in place to slow the spread of coronavirus disease 2019 (COVID-19), as the incidence of IMD has since increased to 0.12/100,000 people in 2022.2
Most IMD is caused by five of the 12 meningococcal serogroups, namely A (MenA), B (MenB), C (MenC), W (MenW), and Y (MenY).3 In Argentina in 2007, approximately 66% of IMD isolates belonged to MenB, while around 13% belonged to MenW.4,5 From 2007 to 2012, the proportion of MenB decreased while MenW increased,5 reaching approximately 36% and 57%, respectively, in 2012.2 After 2012, the proportion of MenB increased, while MenW decreased, with MenB becoming predominant in 2015.2 In 2022, MenB accounted for around 65%, MenW had decreased to approximately 15%, and MenC and MenY accounted for around 12% and 6%, respectively.2
Tetravalent MenA, MenC, MenW, MenY (MenACWY) vaccine (Menveo, GSK) was introduced into the Argentinian National Immunization Program (NIP) in 2017 at ages 3, 5, and 15 months and 11 y.6 Four-component MenB (4CMenB, Bexsero, GSK) vaccine has been recommended since 2020 in Argentina for people at increased risk of IMD, e.g. patients with asplenia, complement deficiency, or receiving eculizumab.7
4CMenB vaccine contains Neisseria meningitidis group B Neisserial Heparin Binding Antigen (NHBA), Neisseria adhesin A (NadA), and factor H binding protein (fHbp); and outer membrane vesicles derived from MenB strain NZ98/254 (OMV NZ), the immunodominant component of which is porin A (PorA) 1.4. As these antigens can be present and conserved in non-serogroup B meningococci, the 4CMenB vaccine could potentially induce protective antibodies against other meningococcal serogroups.8–10
The objective of the current study was to evaluate the ability of 4CMenB-induced antibodies to kill – in human serum bactericidal assay (hSBA) – representative MenW strains responsible for IMD in Argentina during the epidemiological years from January 2010 to December 2011, with the ultimate aim of understanding the impact that 4CMenB vaccination could have on MenW disease. Strains were selected as representative based on multi-locus sequence typing (MLST) and 4CMenB antigen repertoire.
Twenty-four epidemiologically representative (based on date, geographical area, age group, sex, pathology, and clonal complex) MenW isolates were selected from a total of 137 isolates obtained from pediatric and adult patients with IMD, of which 22 were viable. These isolates were obtained during 2010–2011 from the laboratories of hospitals and health centers from the Argentinian National Laboratory Network. Table S1 details the sources of the 22 viable isolates (i.e. region, age, sex, disease, etc.), which were all from different patients. These were characterized by MLST by Sanger Technology in the National Reference Laboratory (NRL) in 2011; and 19 that were still viable in 2022 were sequenced with Illumina technology at ANLIS Malbran. The NRL participates annually in the external quality assurance UK NEQAS (https://ukneqas.org.uk) and was designated as a NRL by Resolution No. 70 (January 21, 2014) of the Ministry of Health. This laboratory participates as a NRL of Argentina in the SIREVA network, coordinated by the Pan American Health Organization. FastQC was used to quality control the fastq files.11 Short-reads were assembled in contigs by Unicycler v0.4.8-beta.12 Genome sequences were analyzed with the PubMLST Neisseria database13 and automatically characterized by the BIGSdb platform at finetyping loci (porA, ferric enterochelin receptor A [fetA]), MLST genes (putative ABC transporter [abcZ], adenylate kinase [adk], shikimate dehydrogenase [aroE], fumarate hydratase [fumC], glucose-6-phosphate dehydrogenase [gdh], pyruvate dehydrogenase subunit [pdhC], phosphoglucomutase [pgm]) and were molecularly characterized for 4CMenB vaccine antigens.
Overall, 18 of 22 strains (81.8%) belonged to sequence type (ST)-11, of which 17 strains carried PorA 5,2 and one carried PorA 7,30 (neither of which matched the PorA 1.4 in 4CMenB vaccine) (Table S2 and central bars of each part of Figure 1). Three strains belonged to ST-22, all of which carried PorA 18–1,3; one strain belonged to ST-1768 and carried PorA 21,4. All 22 strains harbored fHbp variant 2 (mismatched to fHbp variant 1.1 in 4CMenB vaccine), of which 16 strains had fHbp variant 2.22, three had 2.16, and fHbp variants 2.160, 2.346, and 2.869 were harbored by one isolate each. Fourteen strains had NHBA peptide variant 29, two had variant 20, one had variant 53 (none of which matched NHBA peptide 2 in 4CMenB vaccine), one had no NHBA peptide present, and for four strains, this was not determined. Sixteen strains had NadA peptide 6 (which corresponds to variant 2/3 of 4CMenB vaccine), three strains did not have NadA peptide, and for three strains, this was not determined.
Figure 1.
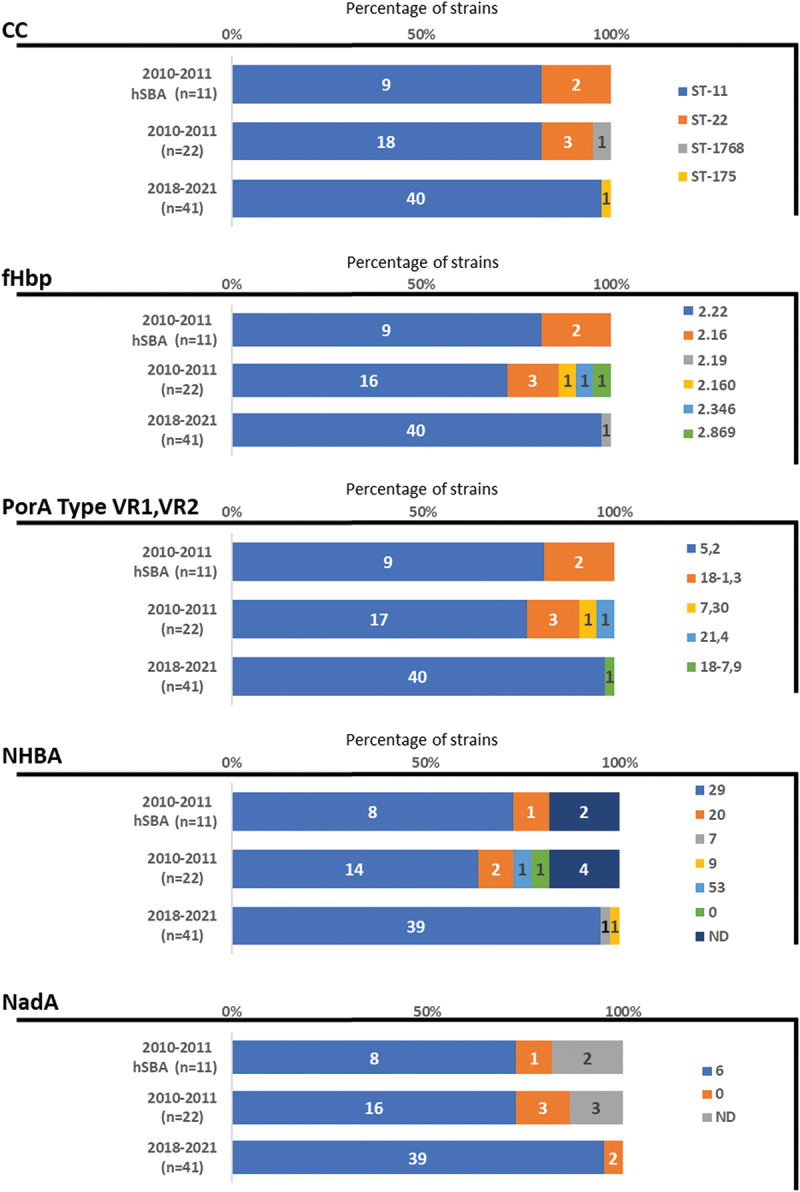
Characteristics of the 11 hSBA-tested MenW strains from 2010–2011; all 22 strains from 2010–2011; and the 41 strains from 2018–2021.
Abbreviations: 0, not present; CC, clonal complex; fHbp, factor H binding protein; hSBA, human serum bactericidal assays; MenW, meningococcal serogroup W; n, number; NadA, Neisseria adhesin A; ND, not determined; NHBA, Neisserial Heparin Binding Antigen; porA, porin A; ST, sequence type; VR, variable region.
From the 22 samples, 11 MenW isolates that were considered representative (based on MLST and 4CMenB vaccine antigen repertoire) of Argentinian samples from 2010–2011 were tested in standardized human serum bactericidal assays (hSBA). Nine of the 11 strains belonged to ST-11 and had PorA 5,2 and fHbp variant 2.22. Of these nine, eight had NHBA peptide variant 29 and NadA peptide 6, while NHBA and NadA information was not available for the other one (Table S2 and top bars of each part of Figure 1). The two remaining strains belong to ST-22 and had PorA 18-1,3, and fHbp variant 2.16. One of the ST-22 strains had NHBA peptide 20 and no NadA, while NHBA and NadA were not determined for the other. These 11 strains are summarized in Figure 2.
Figure 2.
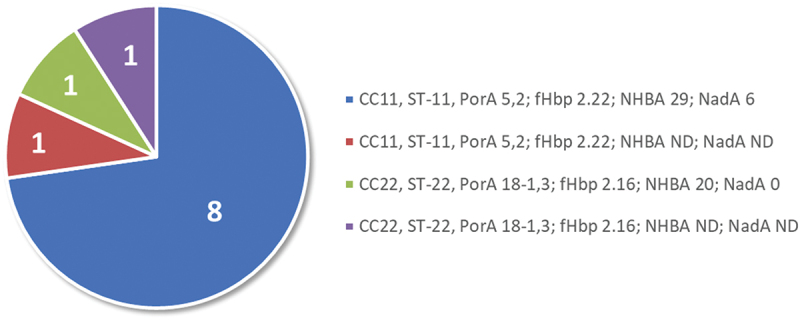
Characteristics of the 11 hSBA-tested MenW strains from 2010–2011.
Abbreviations: 0, not present; CC, clonal complex; fHbp, factor H binding protein; hSBA, human serum bactericidal assays; MenW, meningococcal serogroup W; NadA, Neisseria adhesin A; ND, not determined; NHBA, Neisserial Heparin Binding Antigen; porA, porin A; ST, sequence type.
Three pairs of pooled sera derived from randomly selected subjects were tested, derived from: (1) 10 adolescents (a) before and (b) 1 month after one dose of MenACWY (Menveo; GSK, Belgium) (NCT00518180); (2) 12 adolescents (a) before and (b) 1 month after the second of two doses (2 months apart) of 4CMenB vaccine (NCT00661713); and (3) (a) 180 infants who had not received 4CMenB vaccine (NCT00657709) and (b) 40 infants 1 month after the fourth of four doses (at ages 2, 4, 6, and 12 months) of 4CMenB vaccine (NCT00847145). To evaluate whether responses against MenW were due to a specific 4CMenB vaccine effect, further tests were performed on four isolates using sera that had been incubated with a mixture of the three recombinant antigens and OMV (in the same proportions as in the 4CMenB vaccine, but without alum) at a concentration of 500 µg/mL to deplete 4CMenB vaccine antibodies. Informed consent forms that included permission for potential reuse of biological samples were obtained from subjects or their parent/guardian in the four clinical trials, which were conducted in accordance with Good Clinical Practices and the Declaration of Helsinki, with approval of the protocols by ethics committees of the participating centers. For some participants in study NCT00661713, the collected informed consent did not include permission for reuse of the biological samples, some of which were used in the current study. Upon discovery of this unintended error, GSK informed the principal investigators, the local ethics committee, and the Ministry of Health of the concerned country and notified all impacted participants. The sera samples used in this study were anonymous pools.
All strains were very efficiently killed by antibodies raised by MenACWY, which was used as positive control for the assay (Table 1). The pooled sera of adolescents who had been immunized with two doses of 4CMenB vaccine showed high bactericidal killing against 10 out of the 11 strains tested (90.9%), with bactericidal titers ≥64 (Table 1). The same 10 strains were killed by infant sera at titers >64 (Table 1). As expected, the two pre-immune and the one control sera pools elicited titers at or below the minimum dilution tested for all MenW isolates.
Table 1.
Bactericidal titers by vaccination status.
| MenW isolate | MenACWY vaccine |
4CMenB vaccine |
||||
|---|---|---|---|---|---|---|
| Adolescents |
Adolescents |
Infants |
||||
| Pre-immune | Post-immune | Pre-immune | Post-2nd dose | Control sera | Post-4th dose | |
| ARG-2991 | <16 | 256 | <4 | <4 | <2 | <2 |
| ARG-3006 | <16 | >512 | <4 | >128* | <2 | >64 |
| ARG-3052 | <16 | 256 | <4 | >128 | 2 | >64† |
| ARG-3064 | <16 | 256 | <4 | >128 | <2 | >64 |
| ARG-3158 | <16 | >512 | <4 | 64* | <2 | >64 |
| ARG-3200 | <16 | 256 | <4 | >128 | <2 | >64 |
| ARG-3216 | <16 | 256 | <4 | >128 | <2 | >64* |
| ARG-3223 | <16 | 256 | 4 | >128 | 2 | >64 |
| ARG-3273 | <16 | >512 | <4 | >128* | <2 | >64* |
| ARG-3279 | <16 | ≥512 | <4 | 64 | <2 | >64 |
| ARG-3320 | <16 | ≥512 | <4 | >128* | <2 | >64 |
*Prozone effect: bacterial survival was observed at high antibody concentrations due to the prozone effect, which is often detected when there is an excess of antibodies.
†Bacteriostatic.
4CMenB, 4-component meningococcal serogroup B vaccine; ARG, Argentina; MenACWY, meningococcal serogroups A, C, W, and Y; MenW, meningococcal serogroup W.
To show that the high bactericidal killing measured against the MenW strains was mediated by the specific activity of antibodies induced by immunization with the 4CMenB vaccine, pooled sera from 4CMenB-immunized adolescents and infants were incubated with 4CMenB vaccine antigens to deplete 4CMenB vaccine antibodies. The depleted sera were tested in the hSBA assay. Serum pools that had been depleted of anti-4CMenB vaccine antibodies were no longer able to kill the target strains, with bactericidal titers reduced to baseline levels (Table 2). These data demonstrate that the bactericidal killing of the MenW strains is mediated by antibodies raised by vaccination with 4CMenB.
Table 2.
Bactericidal titers using untreated and 4CMenB antibody-depleted sera.
| MenW isolate | 4CMenB vaccine |
|||
|---|---|---|---|---|
| Adolescents |
Infants |
|||
| Untreated | Depleted* | Untreated | Depleted* | |
| ARG-3006 | >128† | <4 | >64 | <8 |
| ARG-3064 | >128† | <4 | >64 | <8 |
| ARG-3216 | >128 | <8 | >128 | <16 |
| ARG-3273 | >128† | <8 | >128 | <16 |
*Preincubated with 500 µg/mL 4CMenB.
†Prozone effect: bacterial survival was observed at high antibody concentrations due to the prozone effect, which is often detected when there is an excess of antibodies.
4CMenB, 4-component meningococcal serogroup B vaccine; ARG, Argentina; MenW, meningococcal serogroup W.
Of the 10 MenW strains that were killed by the pooled sera, eight had PorA 5,2 (different from the PorA of OMV NZ and therefore not cross-reactive) and fHbp 2.22 (known not to be cross-reactive with fHbp variant 1 present in 4CMenB vaccine).14 Seven of these eight had NHBA variant 29 and NadA antigen 6 (which corresponds to the variant 2/3 in 4CMenB vaccine),15,16 while NHBA and NadA were not determined for the other strain. Based on the mismatch with PorA and fHbp to the variant present in the 4CMenB vaccine, we can envisage that bactericidal killing may be mediated by NadA and NHBA antibodies. However, the only MenW strain that was not killed also had the same NadA and NHBA sequences, suggesting that resistance to killing may be due to additional factors, such as expression levels or OMV antigenic repertoire. We have previously demonstrated that cooperation and synergy of antibodies directed against different antigens play a key role in protection.17 For the strain missing NadA (ARG-3064), killing could be mediated by antibodies targeting one or more antigens acting alone or synergistically, as has already been observed for MenW strains isolated in Europe and Brazil.8,9,18
Also as previously observed,8–10 our findings indicate that the ability of the antibodies induced by 4CMenB antigens to mediate bactericidal killing against meningococcal isolates, including MenW strains, is linked to the presence, sequence diversity, and level of expression of the respective antigens, and is likely mediated by antibodies targeting multiple antigens. This is consistent with the fact that the various tools that have been developed to predict the coverage of 4CMenB against different MenB strains (e.g. Meningococcal Antigen Typing System [MATS]19 and Meningococcal Deduced Vaccine Antigen Reactivity [MenDeVAR] Index20 are not applicable to non-MenB strains. This is probably because of their different antigenic repertoire and the cooperative killing effect of antibodies targeting different antigens that is not measured by these assays.
To evaluate whether there were changes in epidemiology over time, we have recently analyzed 41 out of a total of 48 community-acquired MenW strains from IMD from different patients from hospitals across Argentina during 2018–2021, the sources of which are shown in Table S3. Among these, 40 were characterized as ST-11 and exhibited fHbp peptide 2.22 and PorA 5,2, 39 had NHBA peptide variant 29, and 39 had NadA peptide 6 (Table S4 and bottom bars of each part of Figure 1). One strain was characterized as serotype 5770 (clonal complex ST-175) and exhibited PorA 18–7,9, fHbp 2.19, NHBA 9, and no NadA peptide. As these strains generally had similar genetic profiles to the earlier samples (Figure 1), it is likely that 4CMenB vaccination would still be effective against the MenW strains that were recently circulating in Argentina. Although our results reinforce the evidence of 4CMenB-induced immunity against MenW isolates, a direct extrapolation on coverage of strains from other regions or periods carrying a different combination of antigens would be difficult. Nevertheless, previous observations,8–10,18 along with the current data, indicate that it would be reasonable to expect similar cross-reactivity against MenW isolates in other regions. These data are of interest in view of the incidence of MenW worldwide and the recent emergence and expansion (in Europe) of MenW/ST-11 isolates that may have come from the South American MenW ST-11 isolates.21
In conclusion, 4CMenB vaccination has the potential to protect against MenW disease. The current results show that MenW isolates in Argentina are relatively stable over time, as demonstrated by the clonal complex and 4CMenB vaccine antigenic repertoires. It is expected that 4CMenB vaccine cross-protective ability will also be high against more recent strains. Hence, 4CMenB vaccination could protect against MenW disease in Argentina. Of note, when we started this study, MenACWY vaccine was not recommended in Argentina. Although it has since been included in the Argentinian NIP,6 this program is dynamic and under regular review to take changes in epidemiology and newly available vaccines into account. The introduction of 4CMenB vaccine (instead of MenACWY vaccine) in infants in Argentina is currently being discussed, so our local data on cross protection could be very useful for this decision-making process. Overall, our results support the accumulating evidence8–10,18 that 4CMenB vaccination has the potential to provide cross-protection against MenW disease. Real-world evidence could provide further information on the effectiveness of 4CMenB against non-MenB serogroup disease. Together, this information could help authorities in Argentina and other countries when considering strategies for the prevention of IMD, including updating NIPs or introducing meningococcal vaccines into NIPs.
Supplementary Material
Acknowledgments
The authors thank Maria Gabriela Graña (GSK) for her support during the study and manuscript preparation, and the Argentinian National Laboratory Network for its support to the study. The authors also thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Jenny Lloyd (Compass Medical Communications Ltd., on behalf of GSK) provided medical writing support.
Funding Statement
GlaxoSmithKline Biologicals SA funded this research and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Disclosure statement
Alessia Biolchi, Sara Tomei, and Florencia Nocita are employed by GSK. Mariagrazia Pizza, and Gabriela Vidal were employed by GSK at the time of the study. Mariagrazia Pizza is Professor at Imperial College (London). Alessia Biolchi holds shares in GSK. Josefina Campos was employed by ANLIS Dr. Carlos G. Malbrán at the time of the study. She also received grants from the Pan American Health Organization, Wellcome Trust, and EMBL European Bionformatics Institute; payment/honoraria for lectures from the Wellcome Trust Sanger Institute; and support for attending meetings and/or travel from the Pan American Health Organization, the World Health Organization, and the European Society in Clinical Microbiology and Infectious Diseases. Carla Vizzotti is the current Minister of health in Argentina. These authors declare no other financial or non-financial relationships and activities. Adriana Efron, Cecilia Sorhouet Pereira, Denise De Belder, María Alicia Moscoloni, and Mauricio Santos declare no financial or non-financial relationships and activities and no conflicts of interest.
Author contributors
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Previous congress activities
SADI 2022, 15–17 September 2022, Buenos Aires, Argentina; EMGM 2023, 29 May–1 June 2023, Dubrovnik, Croatia.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2288389.
Trademark
Bexsero and Menveo are trademarks owned by or licensed to GSK.
References
- 1.Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H.. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–6. doi: 10.1016/j.vaccine.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Ministerio de Salud Argentina . Boletín epidemiológico nacional. Número 657. 2023. [accessed 2023 Aug 31]. https://bancos.salud.gob.ar/sites/default/files/2023-06/BEN%20657_SE23_2023.pdf.
- 3.Pardo de Santayana C, Tin Tin Htar M, Findlow J, Balmer P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: a literature review. Epidemiol Infect. 2023;151:e57. doi: 10.1017/S0950268823000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabastou J-M, Agudelo CI, Brandileone MC, Realpe ME, de Oliveira L, de Lemos APS, Valera D, Informe Regional de Sireva II 2007 . Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Washington (DC): Organización Panamericana de la Salud; 2008. [accessed 2023 Jun 2]. https://www.paho.org/es/node/32048. [Google Scholar]
- 5.Gomez JA, Wetzler Malbran P, Vidal G, Seoane M, Giglio ND. Estimation of the real burden of invasive meningococcal disease in Argentina. Epidemiol Infect. 2019;147:e311. doi: 10.1017/S0950268819002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministerio de Salud Argentina . Fundamentos de la introducción de la vacuna tetravalente (ACYW) conjugada contra el meningococo al Calendario Nacional de Inmunizaciones. 2017. [accessed 2023 Sep 6]. https://bancos.salud.gob.ar/recurso/lineamientos-tecnicos-vacuna-meningococo.
- 7.Ministerio de Salud Argentina . Huéspedes especiales: Estrategia de vacunación contra meningococo de Argentina. 2020. [accessed 2023 Sep 6]. https://bancos.salud.gob.ar/recurso/huespedes-especiales-estrategia-de-vacunacion-contra-meningococo-de-argentina.
- 8.Biolchi A, De Angelis G, Moschioni M, Tomei S, Brunelli B, Giuliani M, Bambini S, Borrow R, Claus H, Gorla MCO, et al. Multicomponent meningococcal serogroup B vaccination elicits cross-reactive immunity in infants against genetically diverse serogroup C, W and Y invasive disease isolates. Vaccine. 2020;38(47):7542–50. doi: 10.1016/j.vaccine.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Biolchi A, Tomei S, Brunelli B, Giuliani M, Bambini S, Borrow R, Claus H, Gorla MCO, Hong E, Lemos APS, et al. 4CMenB immunization induces serum bactericidal antibodies against non-serogroup B meningococcal strains in adolescents. Infect Dis Ther. 2021;10(1):307–16. doi: 10.1007/s40121-020-00370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazio C, Biolchi A, Neri A, Tomei S, Vacca P, Ambrosio L, Palmieri A, Mori E, La Gaetana R, Pizza M, et al. Cross-reactivity of 4CMenB vaccine-induced antibodies against meningococci belonging to non-B serogroups in Italy. Hum Vaccin Immunother. 2021;17:2225–31. doi: 10.1080/21645515.2020.1855951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babraham Bioinformatics . FastQC; 2010. [accessed 2022 Nov 25]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 12.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.Org website and their applications [version 1; peer review: 2 approved]. Wellcome Open Res. 2018 Sep 24;3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–54. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bambini S, De Chiara M, Muzzi A, Mora M, Lucidarme J, Brehony C, Borrow R, Masignani V, Comanducci M, Maiden MC, et al. Neisseria adhesin a variation and revised nomenclature scheme. Clin Vaccine Immunol. 2014;21(7):966–71. doi: 10.1128/CVI.00825-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viviani V, Biolchi A, Pizza M. Synergistic activity of antibodies in the multicomponent 4CMenB vaccine. Expert Rev Vaccines. 2022;21:645–58. doi: 10.1080/14760584.2022.2050697. [DOI] [PubMed] [Google Scholar]
- 18.Ladhani SN, Giuliani MM, Biolchi A, Pizza M, Beebeejaun K, Lucidarme J, Findlow J, Ramsay ME, Borrow R. Effectiveness of meningococcal B vaccine against endemic hypervirulent Neisseria meningitidis W strain, England. Emerg Infect Dis. 2016;22:309–11. doi: 10.3201/eid2202.150369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domnich A, Gasparini R, Amicizia D, Boccadifuoco G, Giuliani MM, Panatto D. Meningococcal antigen typing system development and application to the evaluation of effectiveness of meningococcal B vaccine and possible use for other purposes. J Immunol Res. 2015;2015:353461. doi: 10.1155/2015/353461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues CMC, Jolley KA, Smith A, Cameron JC, Feavers IM, Maiden MCJ. Meningococcal deduced vaccine antigen reactivity (MenDevar) index: a rapid and accessible tool that exploits genomic data in public health and clinical microbiology applications. J Clin Microbiol. 2020;59(1):e02161–20. doi: 10.1128/JCM.02161-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucidarme J, Hill DM, Bratcher HB, Gray SJ, du Plessis M, Tsang RS, Vazquez JA, Taha MK, Ceyhan M, Efron AM, et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect. 2015;71:544–52. doi: 10.1016/j.jinf.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


