Abstract
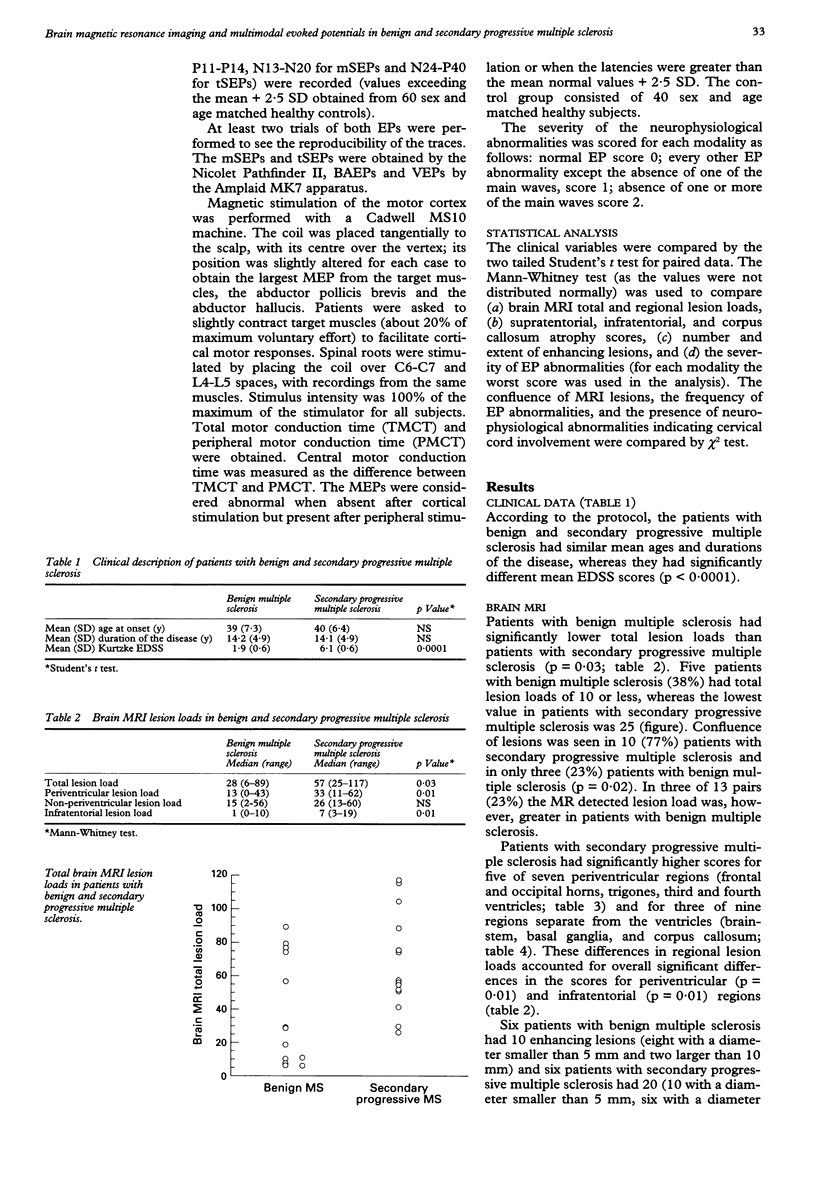
Brain MRI and multimodal evoked potentials (EPs) were obtained for 13 patients with benign multiple sclerosis and 13 patients with secondary progressive multiple sclerosis, matched for age and duration of the disease, to investigate the nature of the disability in multiple sclerosis. Patients with secondary progressive multiple sclerosis had significantly greater lesion loads for five of seven periventricular regions and for three of nine regions separate from the ventricles. Patients with secondary progressive multiple sclerosis also had more severe infratentorial atrophy scores (p = 0.04), whereas there were no differences between the two groups in number and extent of enhancing lesions. The frequencies were significantly higher and severities greater for multimodal EP abnormalities of all the modalities in patients with secondary progressive multiple sclerosis. At least one EP component was absent in 12 (92%) patients with secondary progressive multiple sclerosis but in only one patient (8%) with benign multiple sclerosis (p < 0.001). There was neurophysiological evidence for cervical cord involvement in eight (61%) patients with secondary progressive multiple sclerosis and in one with benign multiple sclerosis (p < 0.01). These data indicate that the total amount of lesions, the distribution, and the nature of the pathological process might all account for the development of disability in multiple sclerosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W. Pathology of multiple sclerosis: progression of the lesion. Br Med Bull. 1977 Jan;33(1):15–20. doi: 10.1093/oxfordjournals.bmb.a071388. [DOI] [PubMed] [Google Scholar]
- Allen I. V., McKeown S. R. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci. 1979 Mar;41(1):81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- Barnes D., Munro P. M., Youl B. D., Prineas J. W., McDonald W. I. The longstanding MS lesion. A quantitative MRI and electron microscopic study. Brain. 1991 Jun;114(Pt 3):1271–1280. doi: 10.1093/brain/114.3.1271. [DOI] [PubMed] [Google Scholar]
- Baumhefner R. W., Tourtellotte W. W., Syndulko K., Waluch V., Ellison G. W., Meyers L. W., Cohen S. N., Osborne M., Shapshak P. Quantitative multiple sclerosis plaque assessment with magnetic resonance imaging. Its correlation with clinical parameters, evoked potentials, and intra-blood-brain barrier IgG synthesis. Arch Neurol. 1990 Jan;47(1):19–26. doi: 10.1001/archneur.1990.00530010027014. [DOI] [PubMed] [Google Scholar]
- Beatty W. W., Goodkin D. E., Monson N., Beatty P. A. Cognitive disturbances in patients with relapsing remitting multiple sclerosis. Arch Neurol. 1989 Oct;46(10):1113–1119. doi: 10.1001/archneur.1989.00520460103020. [DOI] [PubMed] [Google Scholar]
- Comi G., Martinelli V., Medaglini S., Locatelli T., Filippi M., Canal N., Triulzi F., Del Maschio A. Correlation between multimodal evoked potentials and magnetic resonance imaging in multiple sclerosis. J Neurol. 1989 Jan;236(1):4–8. doi: 10.1007/BF00314209. [DOI] [PubMed] [Google Scholar]
- Dousset V., Grossman R. I., Ramer K. N., Schnall M. D., Young L. H., Gonzalez-Scarano F., Lavi E., Cohen J. A. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology. 1992 Feb;182(2):483–491. doi: 10.1148/radiology.182.2.1732968. [DOI] [PubMed] [Google Scholar]
- Farlow M. R., Markand O. N., Edwards M. K., Stevens J. C., Kolar O. J. Multiple sclerosis: magnetic resonance imaging, evoked responses, and spinal fluid electrophoresis. Neurology. 1986 Jun;36(6):828–831. doi: 10.1212/wnl.36.6.828. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Kartsounis L. D., Miller D. H., Youl B. D., Ron M. A. Clinically isolated lesions of the type seen in multiple sclerosis: a cognitive, psychiatric, and MRI follow up study. J Neurol Neurosurg Psychiatry. 1992 Oct;55(10):869–876. doi: 10.1136/jnnp.55.10.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Barker G. J., Horsfield M. A., Sacares P. R., MacManus D. G., Thompson A. J., Tofts P. S., McDonald W. I., Miller D. H. Benign and secondary progressive multiple sclerosis: a preliminary quantitative MRI study. J Neurol. 1994 Feb;241(4):246–251. doi: 10.1007/BF00863776. [DOI] [PubMed] [Google Scholar]
- Gass A., Barker G. J., Kidd D., Thorpe J. W., MacManus D., Brennan A., Tofts P. S., Thompson A. J., McDonald W. I., Miller D. H. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol. 1994 Jul;36(1):62–67. doi: 10.1002/ana.410360113. [DOI] [PubMed] [Google Scholar]
- Giesser B. S., Kurtzberg D., Vaughan H. G., Jr, Arezzo J. C., Aisen M. L., Smith C. R., LaRocca N. G., Scheinberg L. C. Trimodal evoked potentials compared with magnetic resonance imaging in the diagnosis of multiple sclerosis. Arch Neurol. 1987 Mar;44(3):281–284. doi: 10.1001/archneur.1987.00520150035017. [DOI] [PubMed] [Google Scholar]
- Haughton V. M., Yetkin F. Z., Rao S. M., Rimm A. A., Fischer M. E., Papke R. A., Breger R. K., Khatri B. O. Quantitative MR in the diagnosis of multiple sclerosis. Magn Reson Med. 1992 Jul;26(1):71–78. doi: 10.1002/mrm.1910260108. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Nelson L. M., Thompson D. S., Burks J. S., Franklin G. M. Neuropsychological findings in relapsing-remitting and chronic-progressive multiple sclerosis. J Consult Clin Psychol. 1985 Feb;53(1):103–110. doi: 10.1037//0022-006x.53.1.103. [DOI] [PubMed] [Google Scholar]
- Kermode A. G., Thompson A. J., Tofts P., MacManus D. G., Kendall B. E., Kingsley D. P., Moseley I. F., Rudge P., McDonald W. I. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990 Oct;113(Pt 5):1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Kidd D., Thorpe J. W., Thompson A. J., Kendall B. E., Moseley I. F., MacManus D. G., McDonald W. I., Miller D. H. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993 Dec;43(12):2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- Koopmans R. A., Li D. K., Grochowski E., Cutler P. J., Paty D. W. Benign versus chronic progressive multiple sclerosis: magnetic resonance imaging features. Ann Neurol. 1989 Jan;25(1):74–81. doi: 10.1002/ana.410250112. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Larsson H. B., Frederiksen J., Kjaer L., Henriksen O., Olesen J. In vivo determination of T1 and T2 in the brain of patients with severe but stable multiple sclerosis. Magn Reson Med. 1988 May;7(1):43–55. doi: 10.1002/mrm.1910070106. [DOI] [PubMed] [Google Scholar]
- Livingstone I. R., Mastaglia F. L., Edis R., Howe J. W. Visual involvement in Friedreich's ataxia and hereditary spastic ataxia. A clinical and visual evoked response study. Arch Neurol. 1981 Feb;38(2):75–79. doi: 10.1001/archneur.1981.00510020033003. [DOI] [PubMed] [Google Scholar]
- Mauguière F., Ibañez V. The dissociation of early SEP components in lesions of the cervico-medullary junction: a cue for routine interpretation of abnormal cervical responses to median nerve stimulation. Electroencephalogr Clin Neurophysiol. 1985 Nov;62(6):406–420. doi: 10.1016/0168-5597(85)90050-4. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Miller D. H., Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992 Aug;18(4):319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Johnson G., Tofts P. S., MacManus D., McDonald W. I. Precise relaxation time measurements of normal-appearing white matter in inflammatory central nervous system disease. Magn Reson Med. 1989 Sep;11(3):331–336. doi: 10.1002/mrm.1910110307. [DOI] [PubMed] [Google Scholar]
- Ormerod I. E., Miller D. H., McDonald W. I., du Boulay E. P., Rudge P., Kendall B. E., Moseley I. F., Johnson G., Tofts P. S., Halliday A. M. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. 1987 Dec;110(Pt 6):1579–1616. doi: 10.1093/brain/110.6.1579. [DOI] [PubMed] [Google Scholar]
- Palo J., Ketonen L., Wikström J. A follow-up study of very low field MRI findings and clinical course in multiple sclerosis. J Neurol Sci. 1988 Apr;84(2-3):177–187. doi: 10.1016/0022-510x(88)90123-2. [DOI] [PubMed] [Google Scholar]
- Paty D. W., Oger J. J., Kastrukoff L. F., Hashimoto S. A., Hooge J. P., Eisen A. A., Eisen K. A., Purves S. J., Low M. D., Brandejs V. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology. 1988 Feb;38(2):180–185. doi: 10.1212/wnl.38.2.180. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Barnard R. O., Kwon E. E., Sharer L. R., Cho E. S. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993 Feb;33(2):137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Stone L. A., Albert P. S., Frank J. A., Martin R., Armstrong M., Maloni H., McFarlin D. E., McFarland H. F. Clinical worsening in multiple sclerosis is associated with increased frequency and area of gadopentetate dimeglumine-enhancing magnetic resonance imaging lesions. Ann Neurol. 1993 May;33(5):480–489. doi: 10.1002/ana.410330511. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., MacManus D. G., Kendall B. E., Kingsley D. P., Moseley I. F., McDonald W. I. Patterns of disease activity in multiple sclerosis: clinical and magnetic resonance imaging study. BMJ. 1990 Mar 10;300(6725):631–634. doi: 10.1136/bmj.300.6725.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., Wicks D., MacManus D. G., Kendall B. E., Kingsley D. P., McDonald W. I. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991 Jan;29(1):53–62. doi: 10.1002/ana.410290111. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Miller D., Youl B., MacManus D., Moore S., Kingsley D., Kendall B., Feinstein A., McDonald W. I. Serial gadolinium-enhanced MRI in relapsing/remitting multiple sclerosis of varying disease duration. Neurology. 1992 Jan;42(1):60–63. doi: 10.1212/wnl.42.1.60. [DOI] [PubMed] [Google Scholar]
- Truyen L., Gheuens J., Van de Vyver F. L., Parizel P. M., Peersman G. V., Martin J. J. Improved correlation of magnetic resonance imaging (MRI) with clinical status in multiple sclerosis (MS) by use of an extensive standardized imaging-protocol. J Neurol Sci. 1990 May;96(2-3):173–182. doi: 10.1016/0022-510x(90)90130-f. [DOI] [PubMed] [Google Scholar]
- Wiebe S., Lee D. H., Karlik S. J., Hopkins M., Vandervoort M. K., Wong C. J., Hewitt L., Rice G. P., Ebers G. C., Noseworthy J. H. Serial cranial and spinal cord magnetic resonance imaging in multiple sclerosis. Ann Neurol. 1992 Nov;32(5):643–650. doi: 10.1002/ana.410320507. [DOI] [PubMed] [Google Scholar]


