Abstract
In the past 50 years, life expectancy has increased by more than 20 years. One consequence of this increase in longevity is the rise of age-related diseases such as dementia. Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60–70% of cases. AD pathogenesis is not restricted to the neuronal compartment but includes strong interactions with other brain cells, particularly microglia triggering the release of inflammatory mediators, which contribute to disease progression and severity. There is growing evidence revealing the diverse clinical benefits of postbiotics in many prevalent conditions, including neurodegenerative diseases. Here, we tested the ability of bacterial conditioned media (BCM) derived from selected lactic acid bacteria (LAB) strains to regulate core mechanisms relevant to AD pathophysiology in the microglia cell line BV-2. Levilactobacillus brevis CRL 2013, chosen for its efficient production of the neurotransmitter GABA, and Lactobacillus delbrueckii subsp. lactis CRL 581, known for its anti-inflammatory properties, were selected alongside Enterococcus mundtii CRL 35, a LAB strain that can significantly modulate cytokine production. BCM from all 3 strains displayed antioxidant capabilities, reducing oxidative stress triggered by beta-amyloid oligomers (oAβ1–42). Additionally, BCM effectively mitigated the expression of inflammatory cytokines, namely, TNF-α, IL-1β, and IL-6 triggered by oAβ1–42. Furthermore, our study identified that BCM from CRL 581 inhibit the activity of acetylcholinesterase (AChE), a crucial enzyme in AD progression, in both human erythrocytes and mouse brain tissues. Notably, the inhibitory effect was mediated by low-molecular-weight components of the BCM. L. delbrueckii subsp. lactis CRL 581 emerged as a favorable candidate for production of postbiotics with potential benefits for AD therapy since it demonstrated potent antioxidant activity, reduction of cytokine expression, and partial AChE inhibition. On the other hand, E. mundtii CRL 35 showed that the antioxidant activity failed to inhibit AChE and caused induction of iNOS expression, rendering it unsuitable as a potential therapeutic for AD. This study unveils the potential benefits of LAB-derived postbiotics for the development of new avenues for therapeutic interventions for AD.
Introduction
Alzheimer’s disease (AD) is a degenerative disorder of the central nervous system (CNS) that results in a progressive deterioration of cognitive function and severe personality disorders. Post-mortem examination of AD brains reveals neuronal and synaptic loss, amyloid plaques, and neurofibrillary tangles.1 However, there is no real correlation between the levels of cortical plaques and AD-related cognitive impairment. Neuron degeneration at the nucleus basalis of Meynert, which is the origin of most of the cholinergic projections to the neocortex, occurs early in the course of the disease, paralleling cognitive decline.2,3 The importance of the cholinergic system in AD is undeniable and proof of that is the fact that anticholinesterase inhibitors together with memantine (a noncompetitive NMDA receptor antagonist) are the only drugs approved for the treatment of AD.4,5 However, the classical inhibitors constitute only a palliative treatment since the degeneration of the cholinergic system is a consequence rather than the cause of AD.
Recent evidence suggests that vascular pathologic alterations and blood–brain barrier disruption may be pivotal in the neurodegenerative process with associated neuroinflammation as a key player in the onset and development of the disease.6 In fact, brain microvessels from Alzheimer’s patients produce higher amounts of cytokines, which certainly contributes to neuronal damage.7 Neuroinflammation is characterized by oxidative stress and the rise of activated microglia that release neurotoxic compounds and proinflammatory cytokines, which perpetuates the inflammatory state that eventually leads to neuronal damage.8
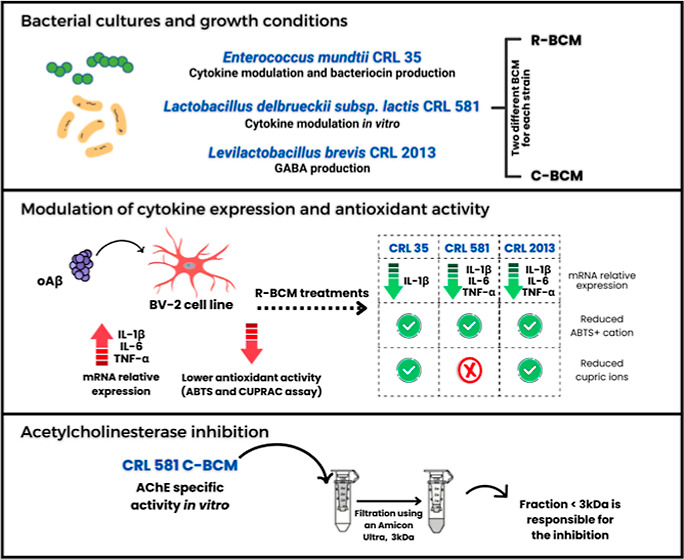
Besides the inhibitors of acetylcholinesterase (AChE) and memantine, the current medical treatment for Alzheimer’s patients includes antidepressants, anxiolytics, neuroleptics, and anti-inflammatory drugs.9 The search for simpler treatments is important to alleviate the burden of the present schemes. A promising alternative is constituted by lactic acid bacteria (LAB).10 Certain LAB strains have been increasingly marketed as probiotic or postbiotic bacteria. The term probiotic refers to live microorganisms, whereas postbiotics consist of nonviable microorganism preparations, including their metabolites and cellular components that, when administered in adequate amounts, exert a beneficial effect on the host’s health.11 In recent years, the potential role of probiotics in neurotherapy discoveries against neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, has been highlighted.10 Nimgampalle and Kuna12 described the restoration of acetylcholine (ACh) levels and improvement in cognitive deficits with Lactiplantibacillus plantarum MTCC1325. On the other hand, supplementation with a blend of Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum optimized hippocampus-dependent spatial memory and synaptic plasticity in rats treated with Aβ.13 Moreover, positive effects of probiotic consumption on improving certain inflammatory and oxidative stress markers in AD patients have been observed.14 Among postbiotic metabolites produced by LAB, some of the better known include B-vitamins, short-chain fatty acids (acetate, propionate, and butyrate), antimicrobial peptides such as bacteriocins and neurotransmitters such as γ-aminobutyric acid (GABA).15,16 Thus, postbiotic metabolites have many health-regulating functions in the body, including absorption of nutrients, detoxification, regulation of the immune system, and microbiota-gut-brain communication. The administration of postbiotics may decrease the synthesis of pro-inflammatory cytokines, hence reducing inflammation and oxidative stress.17 These changes have the potential to ameliorate the effects of senescence and slow down the progression of neurodegenerative diseases such as AD.18 Therefore, the aim of this study was to evaluate the potential of postbiotics derived from three LAB strains to mitigate oxidative stress, modulate cytokine secretion induced by Aβ oligomers, and inhibit AChE activity, which are key aspects of AD pathology. Levilactobacillus brevis CRL 2013 was chosen due to its efficient production of GABA,16,19 a key neurotransmitter that plays a significant role in AD. Importantly, microglia express receptors for neurotransmitters such as GABA and glutamate.20 Additionally, Lactobacillus delbrueckii subsp. lactis CRL 581 was included, because of its ability to reduce the expression of inflammatory cytokines in porcine intestinal epithelial cells and modulate cytokine expression in RAW 264.7, a macrophage-like cell line.21Enterococcus mundtii CRL 35 was the third strain chosen for this study due to its marked modulation of the cytokine profile produced by enterocytes in a listeriosis model (Saavedra, unpublished results). Recent evidence points out that bacterial or viral infections might be associated with vascular alterations in the onset of AD.22 Therefore, besides its ability to modify the expression of cytokines, E. mundtii CRL 35 was selected for its production of the potent cationic peptide called enterocin CRL 35, which has been shown to display antibacterial and antiviral activity.23
Results and Discussion
Bacterial-Conditioned Media Increase Antioxidant Capacity in BV-2 Cells
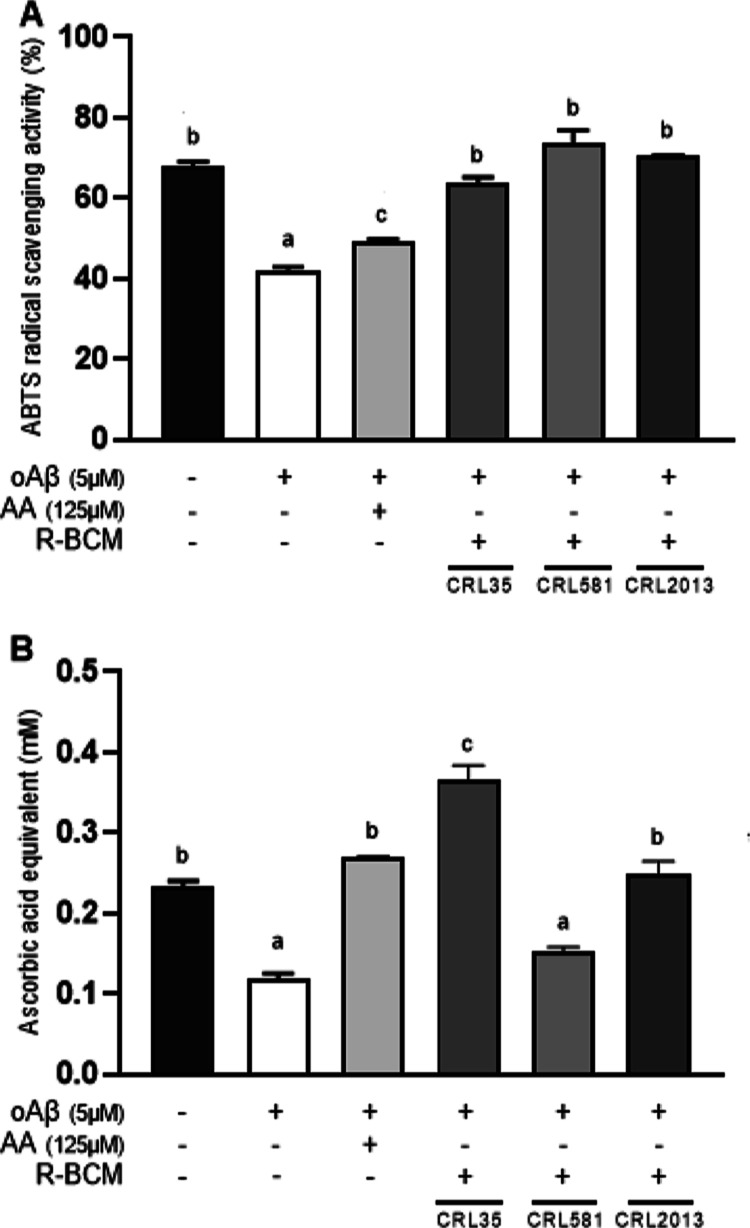
The mouse microglial cell line (BV-2) has been extensively characterized and utilized as a substitute for primary cell cultures in numerous previous studies examining mouse microglial functions, neuroinflammation, and neurodegenerative diseases.24 We assessed the antioxidant activity of the bacterial-conditioned media (BCM) from selected LAB strains (CRL 2013, CRL 581, and CRL 35) in BV-2 cells challenged with oAβ1–42 (Figure 1). As expected, oAβ1–42 caused a reduction of the antioxidant activity measured in supernatants from BV-2 cells, which was efficiently counteracted by ascorbic acid (AA) (Figure 1A). When RPMI-bacterial condition media (R-BCM) from LAB strains were coincubated with oAβ1–42, all supernatants were capable of reducing ABTS + cations (Figure 1A). This result suggests that the three LAB strains analyzed in this study secrete antioxidant metabolites that can significantly reduce the oxidative stress triggered by oAβ1–42 in the microglial cell line.
Figure 1.
BCM from LAB strains cause an antioxidant response from BV-2 cells. BV-2 cells were challenged with oAβ1–42 5 μM for 8 h in the absence or presence of different R-BCM. The antioxidant activity was measured using (A) the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) decolorization assay, and (B) the CUPric Reducing Antioxidant Capacity (CUPRAC) . Ascorbic acid (AA) (125 μM) was used as a positive control. CUPRAC antioxidant activity was expressed as AA equivalents. Data represent the means ± standard error of three independent experiments. Means for each bar without a common letter differ significantly. Significance with Tukey’s HSD posthoc test following a one-way ANOVA is indicated as p < 0.05.
The CUPRAC assay confirmed the oxidative burst triggered by oAβ1–42, as the antioxidant level in the culture medium decreases upon the addition of the oligomers. In this assay, AA (125 μM) was able to reduce cupric ions in the supernatant of oAβ1–42-treated cells (Figure 1B). Interestingly, only R-BCM samples from CRL 35 and CRL 2013 efficiently reduced cupric ions, highlighting their superior capability to mitigate oxidative stress markers produced by beta-amyloid oligomers in vitro (Figure 1B). This finding demonstrates that specific LAB strains, particularly E. mundtii CRL 35 and L. brevis CRL 2013, have the capacity to alleviate the oxidative response induced by oAβ1–42 in microglia; thereby preventing harmful effects on neighboring neurons.25
LAB Postbiotics Modulate Inflammatory Cytokines Expression
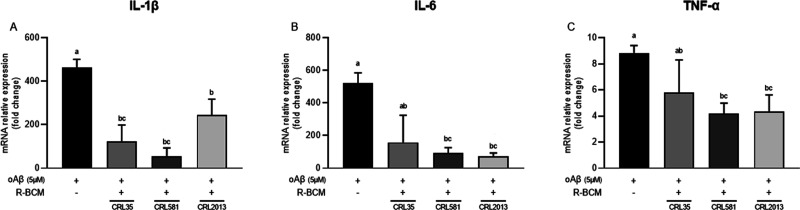
The inflammatory process initiated by activated microglia is another essential component of AD.26 Aggregates of Aβ exhibit significant neurotoxicity by harming neurons. Moreover, these Aβ aggregates stimulate microglia, which eliminate Aβ, but this action leads to the release of inflammatory mediators, resulting in indirect neurotoxic effects.27 Hence, we examined the expression of inflammatory cytokines in BV-2 cells when exposed to oAβ1–42, both in the presence and absence of BCM. Microglial cells challenged with oAβ1–42 exhibited increased expression of cytokines TNF-α, IL-1β, and IL-6 (Figure 2). When cells were coincubated with AA (125 μM) and oAβ (5 μM), no changes in the IL1β expression level were detected (Supporting Information, Figure S1). Conversely, our results showed that treatments with R-BCM from the three selected strains significantly reduced the level of IL-1β (Figure 2A). IL-1β is among the earliest cytokines detected well beyond the normal range in AD.28 It has a well-known negative effect on AD as it strongly induces Aβ peptide production.29 Conversely, Aβ peptide increases the processing of IL-1β in microglia, leading to the overexpression of this cytokine in AD.30 Moreover, R-BCM from CRL 581 and CRL 2013 significantly reduced the level of expression of IL6 (Figure 2B). Even though this cytokine is believed to have a dual role in neurons, i.e., it can either increase neuronal survival or promote neuronal degeneration, the latter effect appears to prevail in AD. However, IL-6 might induce microglial gliosis enhancing the phagocytosis of oAβ and plaque clearance.31 Another important cytokine is TNF-α, which plays a central role in the pathophysiology of AD, being one of the first cytokines that triggers and maintains the inflammatory state.32 Notably, TNF-α expression was significantly reduced in BV-2 cells treated with R-BCM from L. delbrueckii subsp. lactis CRL 581 and L. brevis CRL 2013, underscoring the effectiveness of R-BCM from both strains (Figure 2C). Combining these findings, we can postulate that LAB-conditioned media may exhibit dual effects: possessing both antioxidant and anti-inflammatory properties.
Figure 2.
Postbiotics from selected LAB strains reduced the expression of IL-1β (A), IL-6 (B), and TNF-α (C) in BV-2 cells stimulated by oAβ1–42 5 μM for 8 h. Means for each bar without a common letter differ significantly. Significance with Tukey’s HSD posthoc test following a one-way ANOVA is indicated as p < 0.05.
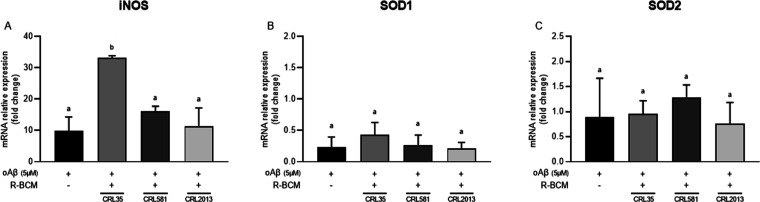
AD-activated microglia often produce excessive amounts of inducible nitric oxide synthase (iNOS), leading to increased NO production, oxidative stress, and neuroinflammation.8 Superoxide dismutase 1 and 2 (SOD-1 and SOD-2, respectively) are antioxidant enzymes that play a vital role in protecting cells from oxidative stress. Understanding the effect of R-BCM on these markers in the context of AD is essential for developing targeted therapies to alleviate the disease’s progression and associated symptoms. Our results showed that E. mundtii CRL 35 induced a significant increase in the expression of iNOS (Figure 3A). Increased iNOS levels have been shown to promote and intensify oxidative stress and favor the production of peroxynitrite, which leads to a progressive cycle of neuroinflammation.33 Conversely, no changes in the expression of SOD-1 and SOD-2 were observed with any of the R-BCM evaluated (Figure 3B,C).
Figure 3.
Effect of postbiotics from selected LAB strains on iNOS (A), SOD-1 (B), and SOD-2 (C) expression in BV-2 cells stimulated by oAβ1–42 5 μM for 8 h. Data represent means ± standard error of three independent experiments. Means for each bar without a common letter differ significantly. Significance with Tukey’s HSD posthoc test following a one-way ANOVA is indicated as p < 0.05.
L. delbrueckii subsp. lactis CRL 581 Metabolites Inhibit Human Erythrocyte and Mouse Brain AchE in Vitro
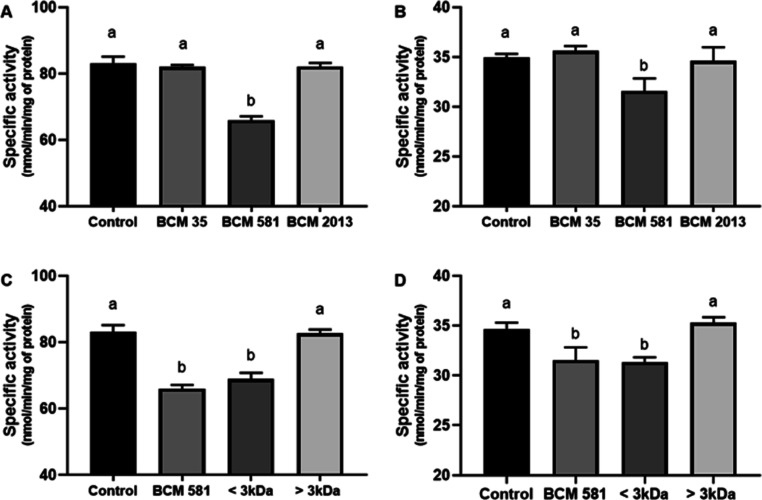
According to the cholinergic hypothesis, the cognitive decline observed in AD is primarily due to disruptions in central cholinergic neurotransmission, which results from a reduction in ACh levels. Cholinesterase inhibitors play a crucial role in improving central cholinergic function by blocking the enzymes responsible for breaking down ACh.34 Donepezil, galantamine, and rivastigmine, as well as huperzine A are the only approved AChE inhibitors for AD management, emphasizing the significance of the cholinergic system as a vital therapeutic target in this pathology. Despite this, there are limited reports on bacterial metabolites inhibiting AChE, and LAB have not been studied in this context.35−37 Therefore, we analyzed the ability of BCM obtained from bacterial strains cultured in a chemically defined medium (C-BCM) to inhibit the AChE activity from human erythrocyte (AChE-E) and mouse brain (AChE-B). Donepezil was used as a control for AChE inhibition (Supporting Information Figure S2). Among the evaluated strains, only the neutralized C-BCM from the CRL 581 strain demonstrated the ability to inhibit both AChE-E and AChE-B (Figure 4A,B). As the majority of AChE inhibitors are typically small molecules, we divided C-BCM extracted from the CRL 581 strain into two fractions using a 3 kDa cutoff membrane and subsequently examined their anticholinesterase activity. Notably, we observed inhibitory effects on both AChE (erythrocytes and mouse brain) when utilizing a fraction with a molecular weight below 3 kDa as illustrated in Figure 4C,D. Notably, the other two strains showed no effect on cholinesterase activity at all in the tested concentrations. These findings suggest that BCM could serve as a promising complement to traditional AD treatment. The presence of small bioactive metabolites in the tested samples suggests that these bioactive molecules might cross the blood–brain barrier and reach the CNS, regardless of the origin of the LAB. This opens up potential avenues for novel therapeutic approaches in addressing AD.
Figure 4.
C-BCM from CRL 581 strain inhibits the specific activity (nmol/min/mg of protein) of AChE-E (A) and AChE-B (B). AChE-E (C) and AChE-B (D) specific activity in the presence of fractioned C-BCM from CRL 581 (<3 and >3 kDa). The results are expressed as mean ± standard deviation of three independent experiments. Means for each bar without a common letter differ significantly. Significance with Tukey’s HSD post hoc test following a one-way ANOVA is indicated as p < 0.05.
Conclusions
Even though initial assessments indicated E. mundtii CRL 35 as a promising candidate, further investigation revealed its unsuitability for AD treatment due to its lack of AChE inhibition and the induction of iNOS expression. In contrast, L. delbrueckii subsp. lactis CRL 581 emerged as the most favorable candidate for AD therapy. This strain not only exhibited highly efficient antioxidant activity but also inhibited the expression of all tested cytokines. Moreover, it partially inhibited AChE activity, which may ameliorate the decrease of cholinergic activity and address underlying pathogenic processes, such as oxidative burst and inflammatory cytokine overexpression.
Although L. brevis CRL 2013 did not exhibit the same level of activity as CRL 581, its unique features (immunomodulatory properties and GABA production) warrant further analysis.16 Ongoing research, in our lab, focusing on studying the effects of L. brevis CRL 2013 in vivo using a C57BL/6 mouse model of mild cognitive impairment holds great promise.
Overall, these findings open up new possibilities for the development of effective AD treatments utilizing bacterially conditioned media from specific LAB strains.
Materials and Methods
Bacterial Cultures, Growth Conditions, and Postbiotic Preparations
The LAB strains used in this study, selected for their beneficial properties, belong to the CERELA culture collection (CERELA-CONICET); E. mundtii CRL 35,23,38Lactobacillus delbrueckii subsp. lactis CRL 581,21,39 and Levilactobacillusbrevis CRL 2013.16 Each LAB strain was cultured in a chemically defined medium (CDM)40 or in a serum-free and antibiotic-free RPMI 1640 medium. Final pH and optical density at 600 nm (OD600nm) were recorded in both culture media (Table 1). Data correspond to at least three independent experiments.
Table 1. Bacterial Strains, Culture Media, Final pH, and OD600nma.
| CDM |
RPMI |
|||
|---|---|---|---|---|
| STRAIN | OD (600 nm) | pH | OD (600 nm) | pH |
| Enterococcus mundtii CRL 35 | 1.70 | 4.63 | 0.62 | 5.94 |
| Lactobacillus delbrueckii subsp. lactis CRL 581 | 1.25 | 4.00 | 0.51 | 6.50 |
| Levilactobacillus brevis CRL 2013 | 2.20 | 5.30 | 0.54 | 6.02 |
CDM; Roswell Park Memorial Institute serum-free and antibiotic-free culture medium.
In order to evaluate the impact of postbiotic preparations on AD events, 24 h cultures from each strain were prepared as follows: the cultures were centrifuged and the supernatants were adjusted to pH 7 using 1 M NaOH, then filtered through a 0.22 μm filter, resulting in the bacterial-conditioned media (BCM) from CDM (C-BCM) or RPMI (R-BCM) for each bacterial strain.
Oligomerization of Amyloid Beta Peptides Aβ1–42
Oligomer Aβ1–42 (oAβ) was prepared according to published protocols48 with some modifications. Briefly, the Aβ1–42 peptide was dissolved in hexafluoroisopropanol (Sigma-Aldrich) to prepare a 1 mM solution that was aliquoted in sterile microcentrifuge tubes. Hexafluoroisopropanol was dried using a speed vacuum concentrator (Biotron Inc., Korea) for 5 min at 30 °C and the peptide was kept at −80 °C. The dried peptide was reconstituted in dimethyl sulfoxide to obtain a 5 mM stock solution. Leibovitz L15-CO2 medium was used to dilute the peptide to a final concentration of 100 μM. This solution was incubated at 4 °C for 24 h prior to the experiments.
Cell Culture
The mouse microglial cell line (BV-2) was generously provided by Dr. Fernando Pitossi from the Leloir Institute Foundation (Argentina). Cells were routinely maintained in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin G (100 U/mL, Biological Industries), streptomycin (0,1 μg/mL, Biological Industries), and amphotericin B (25 μg/mL, Biological Industries) at 37 °C in a humidified 5% CO2-95% air incubator. For each experiment, BV-2 cells were seeded in 6-well plates (8 × 105 cells/mL) and incubated for 24 h. The medium was then replaced with R-BCM from each bacterial strain. To induce an inflammatory state, 5 μM oAβ1–42 was added to each well and samples were collected after 8 h of incubation. RPMI 1640 culture media was used as a control.
Total Antioxidant Capacity
The antioxidant capacity present in BCM and cell-free supernatants from R-BCM-treated BV-2 cells was determined by two methods; the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) decolorization assay and the CUPric Reducing Antioxidant Capacity (CUPRAC) assay. For the ABTS assay,41 a mixture of 7 mM ABTS and 2.45 mM ammonium persulfate was prepared, and the solution was kept in the dark at room temperature for 16 h to produce the ABTS radical cation (ABTS•+) that shows a maximum absorbance at 734 nm. Antioxidants reduce ABTS•+, leading to a decolorization of the solution, whose extent depends on the concentration of the antioxidant. Samples were mixed with ABTS•+ with an initial absorbance of 0.7 at 734 nm (OD734nm), and the final absorbance was read after incubation for 10 min in the dark. The CUPRAC assay is based on the reduction of CUPRAC reagent in the presence of antioxidants.42 The CUPRAC reagent consists of a mixture of 10 mM CuSO4, 7.5 mM neocuproine, and 1 M ammonium acetate buffer, pH 7. The presence of a reducing agent induces the copper (I)-neocuproine complex formation, which has a distinctive absorbance peak at 450 nm. The CUPRAC reagent was incubated for 1 h at room temperature with cell-free supernatants, and the formation of the copper(I)-neocuproine complex formation was estimated by measuring the absorbance at 450 nm. A standard curve was performed with increasing concentrations of AA. The total antioxidant capacity of cell-free supernatants from BV-2 control was subtracted from R-BCM-treated BV-2. In both assays, AA at 125 μM was used as a control.
RT-qPCR
BV-2 adherent cells treated with 5 μM oAβ1–42 in the presence or absence of each R-BCM were incubated for 8 h and then harvested. Total RNA was isolated using RNAzol RT following the manufacturer’s instructions (Molecular Research Center, Inc.). Briefly, cells were lysed in 1 mL of RNAzol RT and 400 μL of RNase-free water was added to each tube. The suspension was centrifuged for 15 min at 12,000g. The RNA-containing supernatants were transferred to new tubes and the RNA was precipitated by adding 1 mL of isopropanol and incubated for 3 h at −20 °C. Samples were centrifuged to discard the supernatants and pellets were washed twice with 70% ethanol. Finally, the RNA pellets were dissolved in 20 μL of RNase-free water and treated with PerfeCTa DNAse I (Quanta Biosciences, Genbiotech, Buenos Aires, Argentina) according to the manufacturer’s protocol. Total RNA purity and quantity were analyzed by Nabi UV/vis Nano Spectrophotometer (MicroDigital Co., Ltd., Korea). cDNA synthesis was carried out using the qScript cDNA SuperMix (Quanta Bio, Genbiotech, Argentina) following the manufacturer’s instructions. An iQ5 Real-Time PCR Detection System (Bio-Rad) with an iQ5 SYBR Green Supermix (Bio-Rad) was used to perform the qPCR. The oligonucleotide primers used are listed in Table 2. The results were expressed as fold changes of the Ct value relative to the untreated cells group using the 2–ΔΔCt method.43 Amplification efficiencies were validated and normalized against those of the β-actin gene. A melting curve analysis was performed immediately at the end of each experiment at a linear temperature transition rate of 0.1 °C/s from 55 to 95 °C to determine the specificity of the amplification.
Table 2. Primer Used for the Quantitative Reverse Transcription Polymerase Chain Reactiona.
| primer
sequence |
|||
|---|---|---|---|
| gene name | forward | reverse | references |
| TNF-α | 5′-GTGGTCAGGTTGCCTCTGTCTC-3′ | 5′-TGGCTCTGTGAGGAAGGCTGTG-3′ | (44) |
| IL-1β | 5′-TTTCCTCCTTGCCTCTGATGGG-3′ | 5′-CCACACGTTGACAGCTAGGTTC-3′ | (44) |
| IL-6 | 5′-CTTGGGACTGATGCTGGTGACA-3′ | 5′-GCCTCCGACTTGTGAAGTGGTA-3′ | (44) |
| SOD-1 | 5′-TGCGTGCTGAAGGGCGAC-3′ | 5′-GTCCTGACAACAACCTGGTTC-3′ | (45) |
| SOD-2 | 5′-GGAGCAAGGTCGCTTACAGA-3′ | 5′-GTGCTCCCACACGTCAATC-3′ | (45) |
| iNOS | 5′-GGACGAGACGGATAGGCAGAGA-3′ | 5′-TCTTCAAGCACCTCCAGGAACG-3′ | (44) |
| β-actin | 5′-CGTGAAAAGATGACCCAGATCA-3′ | 5′-ACAGCCTGGATGGCTACGTA-3′ | (16) |
TNF-α, tumor necrosis factor – α; IL-1β, interleukin-1β; IL-6; SOD-1, superoxide dismutase-1; SOD-2, superoxide dismutase-2; iNOS, inducible nitric oxide synthase.
AChE Inhibition
AChE activity was measured in the presence of different C-BCM. AChE from human erythrocytes (AChE-E) and mouse brain (AChE-B) was used for this experiment. These isoforms were obtained as described elsewhere.46 The inhibition of AChE activity was determined by Ellman’s method, using acetylthiocholine (ATC) as the substrate.47 Briefly, AChE-E and AChE-B were diluted in PBS containing 0.33 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (TNB). Then, each C-BCM was added and incubated for 10 min at 37 °C. CDM and polyphenols previously characterized as efficient inhibitors of AChE were used in the control reactions. Reactions were started upon addition of ATC and the absorbance of the 2-nitro-5-thiobenzoate generated was measured at 412 nm at 37 °C in a microplate reader Infinite 200 PRO (Tecan Trading AG, Switzerland). The results were expressed in terms of the specific activity of the enzyme considering the reaction time (5 min) and the molar extinction coefficient of the reaction product (TNB) at 37 °C (13.8 × 103 M–1 cm–1), which yields activity values expressed as nanomol/mL/min. Ultimately, the value is normalized dividing it by the protein content, thereby presenting the results as nanomoles per minute per milligram of protein (specific activity). In order to validate the AChE inhibition assay, donepezil was added at different concentrations and AChE activity was determined (Supporting Information Figure S2). AChE activity was plotted against donepezil concentrations, a sigmoid curve was obtained, and the data were fitted using a four-parametric logistic model, employing the Marquardt–Levenberg algorithm (SigmaPlot 12.0).
Statistics
Unless otherwise indicated, comparisons between the two groups were done via an unpaired t-test. Comparisons between multiple treatment groups were done via a one-way analysis of variance (ANOVA) with Tukey’s multiple comparison post hoc test. All statistical analyses were performed using GraphPad Prism (Version 8).
Acknowledgments
This study was supported by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, and the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06805.
IL1β expression in the presence of AA and dose–response curve of donepezil inhibition on AChE activity from human erythrocytes (AChE-E) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- DeTure M. A.; Dickson D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14 (1), 32. 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. K.; Chang R. C.; Pearce R. K.; Gentleman S. M. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129 (4), 527–540. 10.1007/s00401-015-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T.; Price D. L.; DeLong M. R. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science 1983, 219 (4589), 1184–1190. 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Kandimalla R.; Reddy P. H. Therapeutics of neurotransmitters in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57 (4), 1049–1069. 10.3233/JAD-161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zueva I.; Dias J.; Lushchekina S.; Semenov V.; Mukhamedyarov M.; Pashirova T.; Babaev V.; Nachon F.; Petrova N.; Nurullin L.; et al. New evidence for dual binding site inhibitors of acetylcholinesterase as improved drugs for treatment of Alzheimer’s disease. Neuropharmacology 2019, 155, 131–141. 10.1016/j.neuropharm.2019.05.025. [DOI] [PubMed] [Google Scholar]
- Sweeney M. D.; Kisler K.; Montagne A.; Toga A. W.; Zlokovic B. V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21 (10), 1318–1331. 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V.; Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement 2016, 12 (6), 719–732. 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Andronie-Cioara F. L.; Ardelean A. I.; Nistor-Cseppento C. D.; Jurcau A.; Jurcau M. C.; Pascalau N.; Marcu F. Molecular mechanisms of neuroinflammation in aging and Alzheimer’s disease progression. Int. J. Mol. Sci. 2023, 24 (3), 1869. 10.3390/ijms24031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V.; Antognoli R.; Okoye C.; Monzani F. The use of antipsychotic drugs for treating behavioral symptoms in Alzheimer’s disease. Front. Pharmacol 2019, 10, 1465. 10.3389/fphar.2019.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutalib N. A.; Mohamad S. A. S.; Jusril N. A.; Hasbullah N. I.; Amin M. C. I. M.; Ismail N. H. Lactic acid bacteria (LAB) and neuroprotection, what is new? An up-to-date systematic review. Pharmaceuticals 2023, 16 (5), 712. 10.3390/ph16050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S.; Collado M. C.; Endo A.; Hill C.; Lebeer S.; Quigley E. M. M.; Sanders M. E.; Shamir R.; Swann J. R.; Szajewska H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18 (9), 649–667. 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgampalle M.; Kuna Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11 (8), Kc01–kc05. 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei Asl Z.; Sepehri G.; Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav. Brain Res. 2019, 376, 112183. 10.1016/j.bbr.2019.112183. [DOI] [PubMed] [Google Scholar]
- Akbari E.; Asemi Z.; Kakhaki R. D.; Bahmani F.; Kouchaki E.; Tamtaji O. R.; Hamidi G. A.; Salami M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A.; Pizzolongo F.; De Luca L.; Blaiotta G.; Aponte M.; Addeo F.; Romano R. Production of butyric acid by different strains of Lactobacillus plantarum (Lactiplantibacillus plantarum). Int. Dairy J. 2023, 140, 105589. 10.1016/j.idairyj.2023.105589. [DOI] [Google Scholar]
- Cataldo P. G.; Villena J.; Elean M.; Savoy de Giori G.; Saavedra L.; Hebert E. M. Immunomodulatory properties of a γ-aminobutyric acid-enriched strawberry juice produced by Levilactobacillus brevis CRL 2013. Front. Microbiol. 2020, 11, 3176. 10.3389/fmicb.2020.610016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.; Rodriguez J.; Wee J. Dietary postbiotics reduce cytotoxicity and inflammation induced by crystalline silica in an in vitro RAW 264.7 macrophage model. Foods 2022, 11 (6), 877. 10.3390/foods11060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ağagündüz D.; Kocaadam-Bozkurt B.; Bozkurt O.; Sharma H.; Esposito R.; Özoğul F.; Capasso R. Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: The gut-health axis for a good mind. Biomed. Pharmacother. 2022, 153, 113430. 10.1016/j.biopha.2022.113430. [DOI] [PubMed] [Google Scholar]
- Cataldo P. G.; Villegas J. M.; Savoy de Giori G.; Saavedra L.; Hebert E. M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. 10.1016/j.ijfoodmicro.2020.108792. [DOI] [PubMed] [Google Scholar]
- Logiacco F.; Xia P.; Georgiev S. V.; Franconi C.; Chang Y. J.; Ugursu B.; Sporbert A.; Kühn R.; Kettenmann H.; Semtner M. Microglia sense neuronal activity via GABA in the early postnatal hippocampus. Cell Rep. 2021, 37 (13), 110128. 10.1016/j.celrep.2021.110128. [DOI] [PubMed] [Google Scholar]
- Elean M.; Albarracin L.; Fukuyama K.; Zhou B.; Tomokiyo M.; Kitahara S.; Araki S.; Suda Y.; Saavedra L.; Villena J.; et al. Lactobacillus delbrueckii CRL 581 differentially modulates tlr3-triggered antiviral innate immune response in intestinal epithelial cells and macrophages. Microorganisms 2021, 9 (12), 2449. 10.3390/microorganisms9122449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigasova D.; Nemergut M.; Liskova B.; Damborsky J. Multi-pathogen infections and Alzheimer’s disease. Microb. Cell Factories 2021, 20 (1), 25. 10.1186/s12934-021-01520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra L.; Minahk C.; de Ruiz Holgado A. P.; Sesma F. Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Antimicrob. Agents Chemother. 2004, 48 (7), 2778–2781. 10.1128/AAC.48.7.2778-2781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A.; Lund S.; Hedtjärn M.; Schrattenholz A.; Pörzgen P.; Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 2009, 26 (2), 83–94. 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Simpson D. S. A.; Oliver P. L. ROS Generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9 (8), 743. 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B.; Landreth G. E. Inflammation, microglia, and Alzheimer’s disease. Neurobiol. Dis. 2010, 37, 503–509. 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I.; Zimin P. I.; Wulff H.; Jin L. W. Amyloid-β Protein Oligomer at Low Nanomolar Concentrations Activates Microglia and Induces Microglial Neurotoxicity. J. Biol. Chem. 2011, 286 (5), 3693–3706. 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bona D.; Plaia A.; Vasto S.; Cavallone L.; Lescai F.; Franceschi C.; Licastro F.; Colonna-Romano G.; Lio D.; Candore G.; et al. Association between the interleukin-1β polymorphisms and Alzheimer’s disease: A systematic review and meta-analysis. Brain Res. Rev. 2008, 59 (1), 155–163. 10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Xie L.; Lai Y.; Lei F.; Liu S.; Liu R.; Wang T. Exploring the association between interleukin-1β and its interacting proteins in Alzheimer’s disease. Mol. Med. Rep. 2015, 11 (5), 3219–3228. 10.3892/mmr.2015.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneo J.; Adachi T.; Yoshida A.; Takayasu K.; Takahara K.; Inaba K. Amyloid β oligomers induce interleukin-1β production in primary microglia in a cathepsin B- and reactive oxygen species-dependent manner. Biochem. Biophys. Res. Commun. 2015, 458 (3), 561–567. 10.1016/j.bbrc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P.; Jansen-West K.; Beccard A.; Ceballos-Diaz C.; Levites Y.; Verbeeck C.; Zubair A. C.; Dickson D.; Golde T. E.; Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010, 24 (2), 548–559. 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelová H.; Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 2013, 62 (7), 641–651. 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- Justo A. F. O.; Suemoto C. K. The modulation of neuroinflammation by inducible nitric oxide synthase. J. Cell Commun. Signaling 2022, 16 (2), 155–158. 10.1007/s12079-021-00663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H.; Mesulam M. M.; Cuello A. C.; Farlow M. R.; Giacobini E.; Grossberg G. T.; Khachaturian A. S.; Vergallo A.; Cavedo E.; Snyder P. J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141 (7), 1917–1933. 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangnoi Y.; Sakulkeo O.; Yuenyongsawad S.; Kanjana-opas A.; Ingkaninan K.; Plubrukarn A.; Suwanborirux K. Acetylcholinesterase-inhibiting activity of pyrrole derivatives from a novel marine gliding bacterium, Rapidithrix thailandica. Mar. Drugs 2008, 6 (4), 578–586. 10.3390/md6040578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochu D.; Rothlisberger C.; Taupin C.; Renault F.; Gagnon J.; Masson P. Purification, molecular characterization and catalytic properties of a Pseudomonas fluorescens enzyme having cholinesterase-like activity. Biochim. Biophys. Acta 1998, 1385 (1), 126–138. 10.1016/S0167-4838(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Pandey S.; Sree A.; Sethi D. P.; Kumar C. G.; Kakollu S.; Chowdhury L.; Dash S. S. A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microb. Cell Factories 2014, 13 (1), 24. 10.1186/1475-2859-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacina J.; Suárez N.; Hormigo R.; Fadda S.; Lechner M.; Saavedra L. A genomic view of food-related and probiotic Enterococcus strains. DNA Res. 2016, 24 (1), 11–24. 10.1093/dnares/dsw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elean M.; Albarracin L.; Cataldo P. G.; Londero A.; Kitazawa H.; Saavedra L.; Villena J.; Hebert E. M. New immunobiotics from highly proteolytic Lactobacillus delbrueckii strains: their impact on intestinal antiviral innate immune response. Benef. Microbes 2020, 11 (4), 375–390. 10.3920/BM2019.0198. [DOI] [PubMed] [Google Scholar]
- Hébert E. M.; Raya R. R.; Giori G. S. d. Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004, 49 (5), 341–345. 10.1007/s00284-004-4357-9. [DOI] [PubMed] [Google Scholar]
- Saavedra L.; Mohamed A.; Ma V.; Kar S.; Posse de Chaves E. Internalization of β-amyloid peptide by primary neurons in the absence of apolipoprotein E. J. Biol. Chem. 2007, 282 (49), 35722–35732. 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- Ilyasov I.; Beloborodov V.; Antonov D.; Dubrovskaya A.; Terekhov R.; Zhevlakova A.; Saydasheva A.; Evteev V.; Selivanova I. Flavonoids with glutathione antioxidant synergy: influence of free radicals inflow. Antioxidants 2020, 9 (8), 695. 10.3390/antiox9080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apak R.; Güçlü K.; Özyürek M.; Bektaşoğlu B.; Bener M.. Cupric Ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. In Advanced Protocols in Oxidative Stress II; Armstrong D., Ed.; Humana Press, 2010, pp 215–239. [DOI] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25 (4), 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Jia L.; Jia J. Oxiracetam offers neuroprotection by reducing amyloid β-induced microglial activation and inflammation in Alzheimer’s Disease. Front. Neurol. 2020, 11 (11), 623. 10.3389/fneur.2020.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B.; Zhang S.; Wang Y.; Guo Y. Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed. Pharmacother. 2019, 109, 112–119. 10.1016/j.biopha.2018.10.053. [DOI] [PubMed] [Google Scholar]
- Salazar P. B.; de Athayde Moncorvo Collado A.; Canal-Martínez V.; Minahk C. J. Differential inhibition of human erythrocyte acetylcholinesterase by polyphenols epigallocatechin-3-gallate and resveratrol. Relevance of the membrane-bound form. Biofactors 2017, 43 (1), 73–81. 10.1002/biof.1322. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V. Jr; Feather-Stone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.