Abstract
Purpose
Collectin subfamily member 12, a transmembrane scavenger receptor C-type lectin, is aberrantly expressed in various cancers. However, its physiological role in gastric cancer remains somewhat unclear. This study aimed to investigate the Collectin subfamily member 12 expression pattern in human gastric cancer and its role in gastric cancer progression.
Methods
The Kaplan-Meier method was used for survival analysis. The univariate and multivariate Cox proportional hazards regression models were used to identify independent predictors for progression-free survival and overall survival. The effects of Collectin subfamily member 12 on gastric cancer cell proliferation, migration, invasion, and apoptosis were detected through the cell counting kit-8 assay, colony formation assay, wound healing assay, transwell assay, and flow cytometry analysis, respectively. Additionally, the correlation between Collectin subfamily member 12 expression and immune cell infiltration was analyzed through bioinformatics.
Results
Collectin subfamily member 12 was highly expressed in advanced gastric cancer (T3-T4, pathologic stage III-IV). High Collectin subfamily member 12 expression was correlated with a worse progression-free survival and overall survival in the gastric cancer patients. In vitro, cell line studies revealed that Collectin subfamily member 12 promoted gastric cancer cell proliferation, migration, and invasion and inhibited gastric cancer cell apoptosis. The bioinformatics analysis further demonstrated that the Collectin subfamily member 12 expression level positively correlated with infiltration of several immune cells, such as M2 macrophages, dendritic cells, neutrophils, and regulatory T cells, suggesting that Collectin subfamily member 12 may also play a role in suppressing tumor immune response in gastric cancer.
Conclusions
Collectin subfamily member 12 was identified as a novel predictive marker and target for the clinical treatment of gastric cancer.
Keywords: COLEC12, gastric cancer, migration, invasion, tumor-infiltrating lymphocytes, prognosis
Introduction
Gastric cancer (GC) is among the most common digestive tract cancers. It ranks fifth among malignant tumors and fourth in cancer-related deaths. 1 The prognosis of GC usually depends on the early detection and treatment of malignant tumor hallmarks, such as invasion and metastasis. 2 Gastric cancer is typically characterized by absence of symptoms in its early stages, which leads to its late detection and a low 5-year survival rate of only 20% to 30%. 3 Therefore, understanding the processes of tumorigenesis and progression is of great significance for the development of effective therapeutic strategies. Recent studies have shown that the tumor microenvironment (TME), epithelial–mesenchymal transition, and gene targets are crucial to GC progression.4–6 They directly or indirectly promote tumor growth by activating growth signaling pathways in cancer cells or altering the cellular microenvironment through various pathways. However, our current understanding of the molecular processes of GC invasion and metastasis is still in its early stages. Further research is needed to gain a complete understanding of the GC molecular mechanisms.
Collectins belong to a family of C-type lectins that are involved in host defense by binding to microbial carbohydrate antigens. At the molecular level, collectins include collagen-like sequences and carbohydrate recognition domains. 7 Collectin subfamily member 12 (COLEC12), also known as CL-12, or CL-P1) is a member of the collectin family that is highly expressed as a transmembrane receptor in umbilical cord vascular endothelial cells. COLEC12 functions as a pattern-recognition molecule initiating complement activation through the alternative pathway. 8 Previous studies have identified COLEC12 as being involved in several tumor processes, such as regulation of apoptosis and inflammation in osteosarcoma 9 and metastasis in breast cancer. 10 In addition, a high COLEC12 expression level was detected in cancer-associated gastric stromal cells. 11 However, the potential COLEC12 function and mechanism in GC remain unclear. This study aimed to investigate the COLEC12 expression pattern in human GC and its crucial role in the regulation of proliferation, migration, and apoptosis of GC cells.
Materials and Methods
Selection of Patients and Tissue Microarray
A tissue microarray (TMA) was constructed containing 304 paraffin-embedded GC surgical samples from primary tumors resected from patients admitted to Zhongshan Hospital, Fudan University (Shanghai, China) between January 2013 and January 2021. Patients with chronic systemic diseases and other malignant tumors were excluded. Of the patients, 78 had T1 stage GC, 70 had T2 stage GC, 81 had T3 stage GC, and 75 had T4 stage GC. This retrospective study was approved by the ethics committees of Zhongshan Hospital, Fudan University (Shanghai, China). The requirement of patient consent was waived for this study due to its retrospective nature. However, all patients provided written informed consent before the collection of biological samples.
Cell Culture
The human GC cell lines HGC-27 (PUMC000279), MKN-45 (PUMC000229), MGC-803 (PUMC000660), and AGS (PUMC000480) were purchased from the Cell Resource Center of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Shanghai, China). The normal gastric epithelial cell line GES-1 (KMCC-001-0254) was purchased from Shanghai Kunmeng Biotechnology. All cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (#15140122, Gibco, Thermo Fisher Scientific Inc.) in a Thermo Scientific™ Forma™ incubator (Thermo Fisher Scientific Inc.) at 37 °C with a 5% CO2 atmosphere.
Small Interfering RNA Transfection
A COLEC12-targeting small interfering RNA (siRNA) was synthesized by Obio Technology. A nonsilencing siRNA was designed as a negative control (siNC group). Small interfering RNA was transfected using the INTERFERinTM siRNA transfection reagent (#409-10, Polyplus Transfection). The knockdown efficacy was determined by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis 48 h after transfection.
Plasmid Construction and Transfection
The COLEC12 overexpression plasmid and control plasmid were constructed by Obio Technology. Cells were seeded at a density of 5 × 105/well in a 6-well plate, and the plasmid was transfected when the cells reached 60% to 70% confluence. Plasmids were transfected using the Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer's instructions.
Cell Proliferation
The cells were seeded at a density of 2 × 103/well into 96-well plates containing 100 µL medium per well. To evaluate cell viability, the medium was replaced with 100 µL medium containing 10 µL of cell counting kit-8 (CCK-8) assay reagent (Dojindo Molecular Technologies). After 2 h, the 96-well plates were oscillated for 2 to 5 min. The optical density value of the cell suspension was measured at 450 nm on an Epoch Microplate Spectrophotometer (BioTek Instruments Inc.). Each experiment was measured continuously for 5 days and repeated 3 times.
Colony Formation Assay
The cells were seeded at a density of 5 × 103 cells/well into 6-well plates and cultured for 14 days, replacing the medium every 2 days. To examine colony formation, cells were fixed by adding 2000 µL of 4% paraformaldehyde to each well and stained with 1000 µL of crystal violet staining solution for 20 min. Ultimately, images were captured using an Olympus SZ60 dissecting microscope (Olympus Optical) at 200 × magnification, and colonies were counted using the ImageJ software (version 4.3, National Institute of Health [NIH]). Each experiment was repeated 3 times.
Wound Healing Assay
The transfected cells were seeded into 6-well plates and grown until they reached 90% confluence. Straight scratches were made in the cell monolayer in the middle of the well using 200-µL pipette tips, and the wells were gently washed twice with phosphate-buffered saline (PBS) to remove free-floating cells. Then, the scratch was imaged under a Leica DMIL microscope (Leica Microsystems GmbH) and its width was measured using the Leica Application Suite X software (Leica Microsystems GmbH) at the time of the scratch and 12 h and 24 h after the scratch. For quantification, 3 replicate wells for each condition were analyzed for at least 3 independent experiments
Cell Invasion Assay
Cells (1 × 105/well) in serum-free medium were added to the Matrigel invasion chambers (8-µm well; Corning Inc.), and 600 µL of DMEM (with 30% FBS) was added to the lower chamber. The cells were allowed to invade the lower chamber for 24 h at 37 °C, and the noninvaded cells were removed. Then, the chamber was fixed with 4% paraformaldehyde for 20 min and stained with 0.1% crystal violet solution for 30 min. Eventually, images were captured using an Olympus SZ60 inverted microscope (Olympus Optical) at 200 × magnification, and the number of cells invaded was counted using ImageJ (version 4.3, NIH).
Cell Apoptosis Assay
The cells were seeded at a density of 1 × 105/well into 6-well plates. Cell apoptosis was measured using the Annexin V-Alexa Fluor 488/propidium iodide (PI) Apoptosis Detection Kit (Yeasen Biotechnology Co., Ltd). Briefly, the cells in the 6-well plates were harvested with 0.25% trypsin-EDTA solution, washed twice with cold PBS buffer, and centrifuged at 1300 rpm for 3 min. Then, 100 μL of 1 × binding buffer, 5 μL of Annexin V-Alexa Fluor 488, and 10 μL of PI were added and mixed for 15 min at room temperature in the dark. Subsequently, 400 μL binding buffer was added, mixed, and placed on ice. The cell apoptosis results were determined using a C6 plus flow cytometer (BD Biosciences). The FlowJo software (version 9.4.10, Tree Star) was used for the analysis of the flow cytometry data. For quantification, 3 replicate wells for each condition were analyzed in at least 3 independent experiments. Cisplatin (CDDP, HY-17394) was purchased from Med-Chem-Express.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from the cells using TriZol (Yeasen Biotechnology Co., Ltd). Complementary DNA was synthesized using the iScript cDNA Synthesis kit (BioRad Laboratories). The qRT-PCR was performed using the SYBR Green kit (Bio-Rad Laboratories). The primers used for qRT-PCR were as follows: COLEC12-F:5′-AGCTGGACAGCCGGATAACT-3′, COLEC12-R:5′-CGTTCCACTGCCCAGCATAA-3′; GAPDH-F:5′-ACCACAGTCCATGCCATCAC-3′, GAPDH-R:5′-TCCACCACCCTGTTGCTGTA-3′. The relative mRNA expression was determined through the 2−ΔΔCt method using GAPDH as the internal control.
Western Blot Analysis
The cells were lysed with radioimmunoprecipitation assay buffer containing the protease and phosphatase inhibitor (#P1045, Beyotime Biotechnology) on ice for 30 min. Then, after centrifuging the samples at 14 000 g and 4 °C for 5 min, the supernatant was collected and the protein concentration (total lysate) was determined using the bicinchoninic acid assay (Pierce Chemical Co.). The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 10% polyacrylamide gels. Subsequently, after transferring the separated proteins onto nitrocellulose membranes (Bio-Rad Laboratories) and blocking the membranes with 5% skimmed milk for 1 h at room temperature, the membranes were incubated with a primary antibody against COLEC12 (#A10422, 1:500; Abclonal) or GAPDH (#4970, 1:1000; Cell Signaling Technology) overnight at 4 °C. The blots were washed 5 times with TBST (TBS with 0.1% Tween 20) for 5 min each time and incubated with horseradish peroxidase–conjugated secondary antibodies (#ab205718, 1:1000; Abcam) at room temperature for 1 h. After washing, the membrane was visualized using enhanced chemiluminescence solution (MilliporeSigma) and analyzed using the ImageJ software (version 4.3, NIH).
Immunohistochemical Staining
Paraffin-embedded samples were cut into 4 μm tissue sections and processed for IHC staining. The sections were subsequently deparaffinized in xylene and rehydrated in alcohol gradients. After antigen retrieval by microwave pretreatment in citrate buffer (pH 6.0), the sections were sequentially incubated in blocking solution (10% goat serum in PBS, 30 min), anti-COLEC12 primary antibody (#GB112085, 1:500; Servicebio Technology Co., Ltd) overnight at 4 °C, and secondary antibody (#G1213, 1:200, Servicebio Technology Co., Ltd) for 1 h at room temperature. Immunohistochemical staining was evaluated independently by 2 observers based on the staining intensity and the proportion of positively stained cells. The staining intensity was graded as follows: negative = 0, weakly positive = 1, moderately positive = 2, and strongly positive = 3. The proportion of positively stained cells was scored as follows: 1 = 0% to 24% positive cells; 2 = 25% to 49% positive cells; 3 = 50% to 74% positive cells; and 4 = 75% to 100% positive cells. The staining score was determined using the following formula: staining score = staining intensity × proportion of positively stained cells. High COLEC12 expression was defined as a staining score ≥8, while low expression was defined as a staining score <8.
Bioinformatics Analysis
First, the single-sample Gene Set Enrichment Analysis 12 method from the R package “GSVA” (version 1.34.0) 13 was used to determine infiltration enrichment of 24 immune cells in the TME, namely B cells, T cells, CD8 + T cells, T helper (Th) cells, T central memory (Tcm), T effector memory (Tem), T follicular helper (Tfh), T gamma delta (Tgd), Th1, Th17, Th2, regulatory T (Treg) cells, dendritic cells (DCs), activated DCs, immature DCs, plasmacytoid DCs, cytotoxic cells, eosinophils, macrophages, mast cells, neutrophils, NK cells, NK CD56bright cells, and NK CD56dim cells. Based on previously known signature genes of immunocytes, the relative enrichment score was determined from the gene expression profile of each tumor sample. 14 The correlation of COLEC12 expression with several immune marker genes was analyzed using TIMER software (http://cistrome.org/TIMER/). 15 Ultimately, scatter plots were made to analyze the correlations between COLEC12 and macrophage marker genes. The correlation of gene expression with the immune infiltration level was evaluated by Spearman's correlation, and the strength of the correlation was described as follows: 0.00 to 0.19, “very weak”; 0.20 to 0.39, “weak”; 0.40 to 0.59, “moderate”; 0.60 to 0.79, “strong”; and 0.80 to 1.0, “very strong.” 16
Statistical Analysis
The transcriptional expression data of COLEC12 and clinical information of patients with stomach adenocarcinoma (STAD) were downloaded from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases. Level-3 high-throughput sequencing fragments per kilobase per million reads were transformed into transcripts per million and log2 conversion for further analysis. The R package ggplot2 was used to visualize differences in expression. The clinicopathological characteristics of the patients were compared using the Wilcoxon rank-sum test for continuous variables or Pearson χ2 test for rank variables. Kaplan-Meier and Cox regression analyses were performed to evaluate the effect of COLEC12 on survival. The survival package (version 3.2-10) and the survminer package (version 0.4.9) were used for statistical analysis and visualization, respectively. The RMS package (version 6.2-0) was used to construct nomograms and calibration plots. Information about the clinical outcomes of patients, namely progression-free survival (PFS) and overall survival (OS), was obtained from a previous study. 17 Student t test or analysis of variance (ANOVA) was used to analyze the significance of data from CCK-8 assay, colony formation assay, wound healing assay, cell invasion assay, and cell apoptosis assay. Student t test was used for 2-component comparisons and ANOVA test for multicomponent comparisons. For all experiments, at least 3 independent biological replicates of each condition were analyzed, and data were processed with GraphPad Prism (version 7, GraphPad Software). P < .05 was considered statistically significant.
Results
Collectin Subfamily Member 12 Is Highly Expressed in Advanced GC
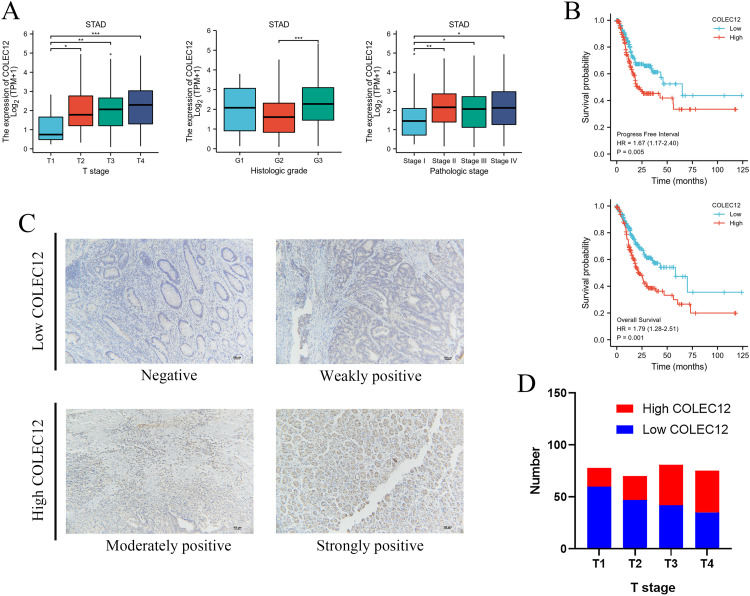
According to data from TCGA, the correlations between COLEC12 mRNA expression levels and relevant clinicopathologic factors including age, gender, TNM stage, pathological stage, and histological grade in the STAD patients were analyzed (Table 1). Collectin subfamily member 12 expression was found to be correlated with T stage (P = .016), pathological stage (P = .010), and histological grade (P < .001) in the STAD patients. The patients with high COLEC12 expression had higher rates of more advanced T stage and pathological stage, and higher histological grade (Figure 1A). Based on the COLEC12 staining intensity (Figure 1C), the IHC analysis of 304 TMA samples revealed that the COLEC12 expression level was also high in the tissue of patients with advanced T-stage GC (Figure 1D).
Table 1.
The Relationship Between COLEC12 Expression and the Clinicopathological Features of Gastric Cancer Patients.a
| Characteristic | Low expression of COLEC12 | High expression of COLEC12 | P value |
|---|---|---|---|
| N | 187 | 188 | |
| Age, n (%) | .880 | ||
| <65 | 83 (22.4%) | 81 (21.8%) | |
| >65 | 102 (27.5%) | 105 (28.3%) | |
| Gender, n (%) | .251 | ||
| Female | 61 (16.3%) | 73 (19.5%) | |
| Male | 126 (33.6%) | 115 (30.7%) | |
| Histological type, n (%) | <.001 | ||
| Diffuse type | 21 (5.6%) | 42 (11.2%) | |
| Mucinous type | 5 (1.3%) | 14 (3.7%) | |
| Not otherwise specified | 103 (27.5%) | 104 (27.8%) | |
| Papillary type | 4 (1.1%) | 1 (0.3%) | |
| Signet ring type | 6 (1.6%) | 5 (1.3%) | |
| Tubular type | 48 (12.8%) | 21 (5.6%) | |
| T stage, n (%) | .016 | ||
| T1 | 15 (4.1%) | 4 (1.1%) | |
| T2 | 47 (12.8%) | 33 (9%) | |
| T3 | 81 (22.1%) | 87 (23.7%) | |
| T4 | 44 (12%) | 56 (15.3%) | |
| N stage, n (%) | .326 | ||
| N0 | 63 (17.6%) | 48 (13.4%) | |
| N1 | 47 (13.2%) | 50 (14%) | |
| N2 | 38 (10.6%) | 37 (10.4%) | |
| N3 | 32 (9%) | 42 (11.8%) | |
| M stage, n (%) | .965 | ||
| M0 | 167 (47%) | 163 (45.9%) | |
| M1 | 12 (3.4%) | 13 (3.7%) | |
| Pathologic stage, n (%) | .010 | ||
| Stage I | 38 (10.8%) | 15 (4.3%) | |
| Stage II | 51 (14.5%) | 60 (17%) | |
| Stage III | 70 (19.9%) | 80 (22.7%) | |
| Stage IV | 19 (5.4%) | 19 (5.4%) | |
| Histologic grade, n (%) | <.001 | ||
| G1 | 4 (1.1%) | 6 (1.6%) | |
| G2 | 90 (24.6%) | 47 (12.8%) | |
| G3 | 88 (24%) | 131 (35.8%) |
Abbreviation: COLEC12, Collectin subfamily member 12.
Numbers in bold are statistically significant (P < .05).
Figure 1.
Relationship between COLEC12 expression and clinicopathological features and prognosis of gastric cancer (GC) patients. (A) Patients with high COLEC12 expression showed higher rates of more advanced T-stage, pathological stage, and higher histological grade. (B) High COLEC12 expression indicated poor progression-free survival (PFS) and overall survival (OS) in GC patients. (C) Immunohistochemical analysis of COLEC12 expression in GC tissue microarrays (TMA). Representative images are shown at 200 × magnification; Scale bar = 100 µm. (D) The expression of COLEC12 in the GC tissues of different T-stages. STAD, stomach adenocarcinoma; *P < .05; **P < .01; ***P < .001. COLEC12, Collectin subfamily member 12.
High COLEC12 Expression Is Correlated With a Worse PFS and OS Rate in GC Patients
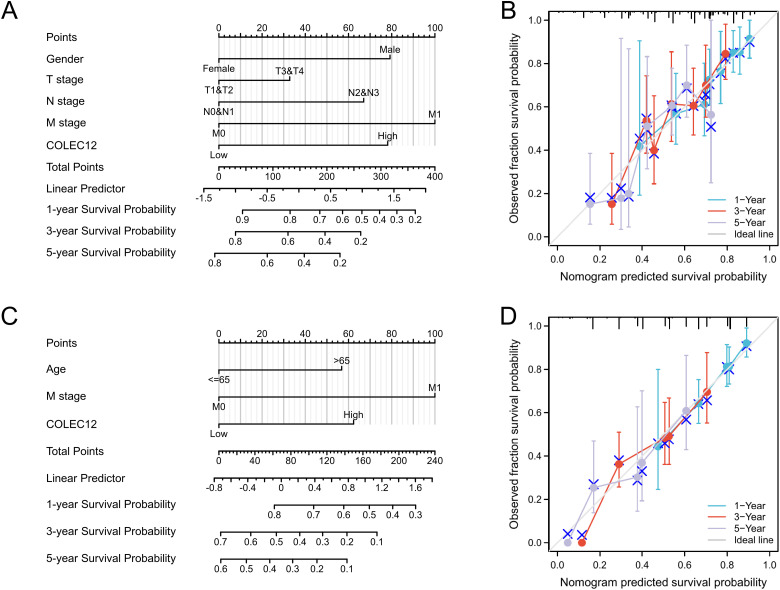
The Kaplan-Meier analysis revealed that high COLEC12 expression in tumor tissues predicted poor PFS and OS in STAD patients (P = .005 and P = .001, respectively), as shown in Figure 1B. According to the Cox univariate analysis, PFS was correlated with gender (P = .015), T stage (P = .018), N stage (P < .001), M stage (P = .012), pathological stage (P = .007), and COLEC12 expression (P = .005) in the STAD patients (Table 2). OS was correlated with age (P = .005), T stage (P = .011), N stage (P = .003), M stage (P = .004), pathological stage (P < .001), and COLEC12 expression (P < .001) (Table 3). A Cox multivariate regression model was constructed to analyze the factors affecting PFS and OS. The male gender (P = .001), higher T stage (P = .046), N stage (P = .005), M stage (P = .011), and COLEC12 expression (P < .001) were independent risk factors for poor PFS (Table 2). Age > 65 (P = .002), higher M stage (P = .006), and COLEC12 expression (P < .001) were independent risk factors for poor OS (Table 3). We also included the independent prognostic factors obtained by the multivariate analysis for the nomogram survival analysis. COLEC12 expression significantly affected PFS and OS compared to the other clinical variables (Figure 2A and C). The bias correction line in the calibration plot was close to the ideal curve (a 45-degree line), which indicated good consistency between the predicted and observed values (Figure 2B and D). We also performed validation studies with data from the GEO database (GSE62254, n = 300), and the results confirmed the effectiveness of our prognostic model (Supplemental Figure 1). Overall, we have constructed a valuable model for predicting survival in GC patients.
Table 2.
The Univariate and Multivariate Cox Proportional Hazard Analyses of Progression-Free Survival for Gastric Cancer Patients According to COLEC12 Expression.a
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 369 | ||||
| <65 | 164 | 1 | |||
| >65 | 205 | 0.858 (0.603-1.221) | .395 | ||
| Gender | 372 | ||||
| Female | 133 | 1 | 1 | ||
| Male | 239 | 1.638 (1.099-2.440) | .015 | 2.017 (1.324-3.074) | .001 |
| T stage | 364 | ||||
| T1&T2 | 97 | 1 | 1 | ||
| T3&T4 | 267 | 1.705 (1.095-2.654) | .018 | 1.752 (1.010-3.038) | .046 |
| N stage | 354 | ||||
| N0&N1 | 205 | 1 | 1 | ||
| N2&N3 | 149 | 1.892 (1.325-2.703) | <.001 | 2.139 (1.262-3.626) | .005 |
| M stage | 353 | ||||
| M0 | 328 | 1 | 1 | ||
| M1 | 25 | 2.224 (1.194-4.144) | .012 | 2.443 (1.229-4.856) | .011 |
| Pathologic stage | 349 | ||||
| Stage I&Stage II | 161 | 1 | 1 | ||
| Stage III&Stage IV | 188 | 1.676 (1.154-2.435) | .007 | 0.687 (0.372-1.268) | .230 |
| Histologic grade | 363 | ||||
| G1 | 10 | 1 | |||
| G2&G3 | 353 | 1.555 (0.384-6.294) | .536 | ||
| COLEC12 | 372 | ||||
| Low | 185 | 1 | 1 | ||
| High | 187 | 1.673 (1.166-2.399) | .005 | 2.031 (1.381-2.986) | <.001 |
Abbreviation: COLEC12, Collectin subfamily member 12.
Numbers in bold are statistically significant (P < .05).
Table 3.
The Univariate and Multivariate Cox Proportional Hazard Analyses of Overall Survival for Gastric Cancer Patients According to COLEC12 Expression.a
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 367 | ||||
| <65 | 163 | 1 | 1 | ||
| >65 | 204 | 1.620 (1.154-2.276) | .005 | 1.784 (1.235-2.578) | .002 |
| Gender | 370 | ||||
| Female | 133 | 1 | |||
| Male | 237 | 1.267 (0.891-1.804) | .188 | ||
| T stage | 362 | ||||
| T1&T2 | 96 | 1 | 1 | ||
| T3&T4 | 266 | 1.719 (1.131-2.612) | .011 | 1.193 (0.704-2.022) | .512 |
| N stage | 352 | ||||
| N0&N1 | 204 | 1 | 1 | ||
| N2&N3 | 148 | 1.650 (1.182-2.302) | .003 | 1.384 (0.874-2.190) | .166 |
| M stage | 352 | ||||
| M0 | 327 | 1 | 1 | ||
| M1 | 25 | 2.254 (1.295-3.924) | .004 | 2.406 (1.290-4.485) | .006 |
| Pathologic stage | 347 | ||||
| Stage I&Stage II | 160 | 1 | 1 | ||
| Stage III&Stage IV | 187 | 1.947 (1.358-2.793) | <.001 | 1.321 (0.752-2.321) | .333 |
| Histologic grade | 361 | ||||
| G1 | 10 | 1 | |||
| G2&G3 | 351 | 1.957 (0.484-7.910) | .346 | ||
| COLEC12 | 370 | ||||
| Low | 184 | 1 | 1 | ||
| High | 186 | 1.788 (1.275-2.507) | <.001 | 1.961 (1.365-2.819) | <.001 |
Abbreviation: COLEC12, Collectin subfamily member 12.
Numbers in bold are statistically significant (P < .05).
Figure 2.
Correlation between COLEC12 expression and other clinical factors with 1-, 3-, and 5-year progression-free survival (PFS) and overall survival (OS) in GC patients. (A) Nomogram for predicting the probability of patients with PFS. (B) Calibration plot of the nomogram for predicting the PFS likelihood. (C) Nomogram for predicting the probability of patients with OS. (D) Calibration plot of the nomogram for predicting the OS. COLEC12, Collectin subfamily member 12; GC, gastric cancer.
Collectin Subfamily Member 12 Knockdown Suppresses GC Cell Proliferation, Migration, and Invasion Abilities and Induces GC Cell Apoptosis
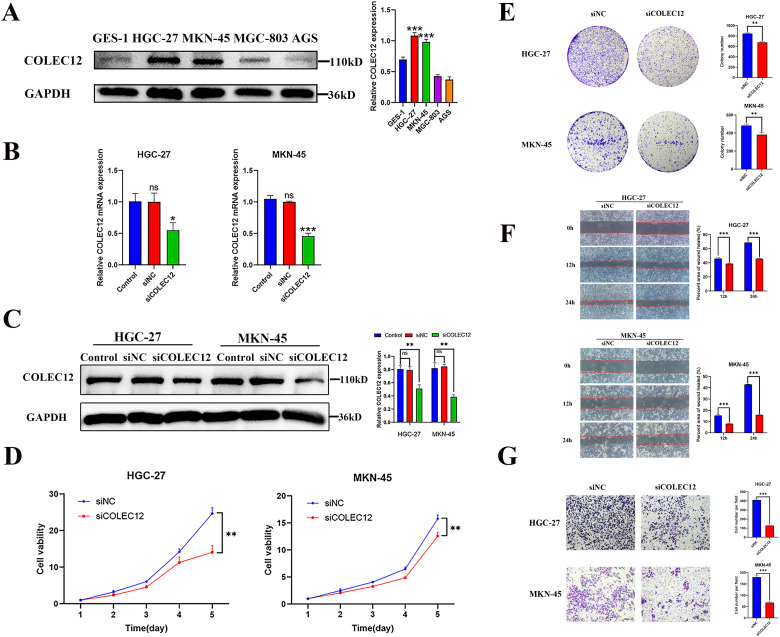
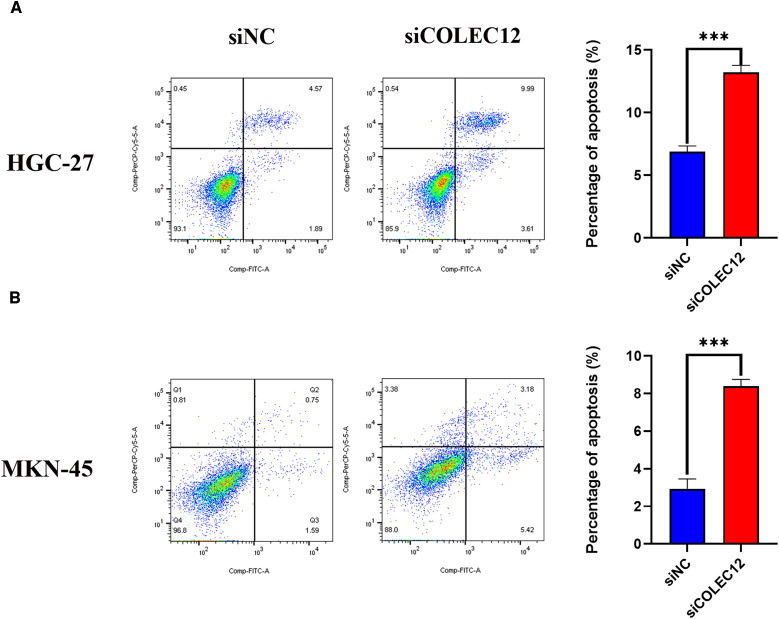
Our examination of COLEC12 expression in the human GC cell lines (HGC-27, MKN-45, MGC-803, and AGS) and the normal gastric epithelial cell line GES-1 revealed that COLEC12 was highly expressed in the HGC-27 and MKN-45 cells (Figure 3A). Based on these results, the HGC-27 and MKN-45 cells were used to verify the functional role of COLEC12. The HGC-27 and MKN-45 cells were transfected with COLEC12-targeting siRNA in the experimental group and with nonsilencing siRNA in the negative control (siNC) group. The knockdown efficiency was verified by qRT-PCR and Western blot analysis. COLEC12 expression was significantly downregulated in the HGC-27 and MKN-45 cells transfected with siCOLEC12 (Figure 3B and C). The cell viability (CCK-8) assay revealed that the GC cell proliferation rate was significantly reduced after COLEC12 knockdown (Figure 3D). The number of GC cell colonies in the siCOLEC12 group was decreased after siRNA transfection compared to that in the siNC group (Figure 3E). In addition, COLEC12 knockdown markedly reduced the migration and invasion abilities compared to the siNC cells, as indicated by a significant decrease in the number of migrated or invaded cells in the siCOLEC12 group (Figure 3F and G). The rate of apoptosis was analyzed 72 h after COLEC12 knockdown by flow cytometry. Annexin V-allophycocyanin (APC) staining revealed that the apoptotic cell rate increased significantly in the siCOLEC12 group compared with the siNC group, suggesting that COLEC12 knockdown induced GC cell apoptosis (Figure 4).
Figure 3.
Knockdown of COLEC12 reduces cell proliferation, migration, and invasion in GC cells. (A) Western blot analysis was performed to detect COLEC12 expression in normal gastric epithelial cell line GES-1 and human GC cell lines (HGC-27, MKN-45, MGC-803, and AGS) (n = 3). (B, C) Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis demonstrated the knockdown of COLEC12 in HGC-27 and MKN-45 cells via transient transfection with siCOLEC12. (D) Cell proliferation assay after COLEC12 knockdown (n = 3). (E) Colony formation assays after the COLEC12 knockdown (n = 3). (F) Migration assay after COLEC12 knockdown (n = 3). (G) Invasion assay after the COLEC12 knockdown (n = 3). Representative images are shown at 200 × magnification; Scale bar = 100 µm. Error bars represent SD. Data represent 3 independent experiments. *P < .05, **P < .01, and ***P < .001. COLEC12, Collectin subfamily member 12; GC, gastric cancer.
Figure 4.
Knockdown of COLEC12 induces apoptosis in GC cells. Flow cytometry analysis was performed to detect and analyze the apoptosis rate of HGC-27 (A) and MKN-45 (B) cells after the COLEC12 knockdown (n = 3). COLEC12, Collectin subfamily member 12; GC, gastric cancer.
Collectin Subfamily Member 12 Overexpression Promotes GC Cell Proliferation, Migration, and Invasion and Inhibits GC Cell Apoptosis
The HGC-27 and MKN-45 cells were transfected with the COLEC12 overexpression plasmids or control plasmids (Supplemental Figure 2A and B). The GC cell proliferation rate and colony number significantly increased after COLEC12 overexpression (Supplemental Figure 2C and D). Also, COLEC12 overexpression markedly increased the cell migration and invasion abilities of GC cells (Supplemental Figure 2E and F). The analysis of the rate of apoptosis 72 h after COLEC12 overexpression by flow cytometry revealed that COLEC12 overexpression reduced the percentage of apoptotic cells in the HGC-27 and MKN-45 cells. In addition, in the presence of the apoptosis-inducing drug cisplatin, the apoptotic cell rate decreased significantly after COLEC12 overexpression (Supplemental Figure 2G).
Collectin Subfamily Member 12 Expression Correlated With the Immune Cell Infiltration Level
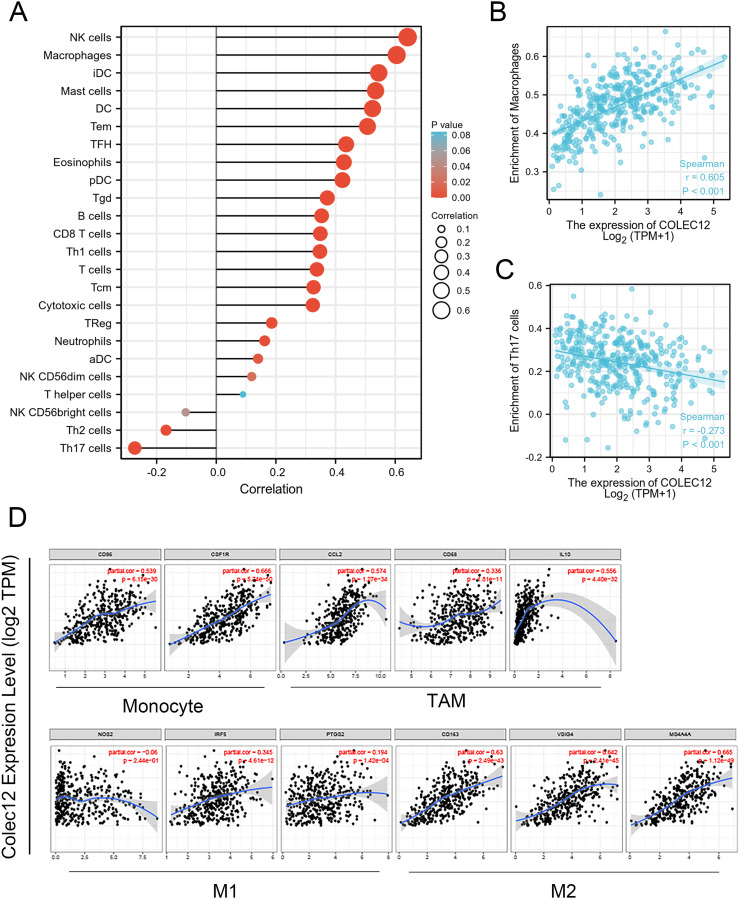
Tumor-infiltrating immune cells are crucial TME components and important predictors of cancer prognosis. 17 In this study, the COLEC12 expression level significantly correlated with the tumor-infiltrating immune cells in GC. COLEC12 expression was significantly positively correlated with infiltration of immune cells, such as NK cells, macrophages, mast cells, and DCs in GC (Figure 5A and B). In addition, COLEC12 expression exhibited a negative correlation with the level of Th17 cell infiltration (Figure 5C). These findings suggest that COLEC12 may play a specific role in infiltration of immune cells in GC, especially of macrophages and Th17 cells.
Figure 5.
Correlation of the COLEC12 expression with immune cell infiltration level in GC. (A) COLEC12 expression had a significant positive correlation with the infiltration level of NK cells, macrophages, mast cells, and DCs. (B) Scatter plots of the correlation between the COLEC12 expression and macrophages. (C) A negative correlation was detected with the infiltration level of Th17 cells. (D) Analysis of the correlation between COLEC12 and the related gene markers of macrophages in GC. Markers include CD86 and CSF1R of monocytes; CCL2, CD68, and IL10 of TAMs; NOS2, IRF5, and PTGS2 of M1 macrophages; and CD163, VSIG4, and MS4A4A of M2 macrophages. Th, T helper cells; Th1, type 1 Th cells; Th2, type 2 Th cells; Th17, type 17 Th cells; Treg, regulatory T cells; Tgd, T gamma delta; Tcm, T central memory; Tem, T effector memory; Tfh, T follicular helper; DCs, dendritic cells; aDCs, activated DCs; iDCs, immature DCs; pDCs, plasmacytoid DCs; NK, natural killer; TAMs, tumor-associated macrophages; COLEC12, Collectin subfamily member 12; GC, gastric cancer.
Correlation Analysis Between COLEC12 Expression and Immune Marker Genes in GC
TIMER is a powerful online tool to analyze immune cell infiltration in different tumors. The correlation between the expression of representative marker genes related to immune cells, including B cells, T cells, monocytes, neutrophils, NK cells, macrophages, and DCs, was analyzed by referring to TIMER databases (Supplemental Table 1). After purity-related adjustment, the results revealed that COLEC12 expression was significantly correlated with the expression of several immune marker genes. High COLEC12 expression in STAD patients was positively correlated with the expression of DC markers, such as HLA-DPB1, HLA-DQB1, HLA-DRA, HLA-DPA1, BDCA-1, BDCA-4, and CD11c; neutrophil markers, such as CD11b; Treg cell markers, such as FOXP3; and T cell depletion markers, such as TIM-3. In addition, COLEC12 expression was found to be significantly correlated with the expression of CCL-2, CD68, and IL10 of tumor-associated macrophages (TAMs); PTGS2 and IRF5 of M1 macrophages; CD163, VSIG4, and MS4A4A of M2 macrophages (Supplemental Table 1). Compared with M1 macrophages, there was a stronger correlation between COLEC12 expression and M2 macrophages (Figure 5D), suggesting that COLEC12 may regulate M2 macrophage polarization in GC.
Discussion
Gastric cancer is among the most common malignancies and one of the leading causes of cancer-related deaths. 18 COLEC12 is located on chromosome 18p11.32 and encodes for a cell surface glycoprotein. The COLEC12 glycoprotein can recognize microbial carbohydrate antigens and bind to them for their clearance. 7 Some studies have found that COLEC12 participates in several tumor processes.9,11 However, the potential function and mechanism of COLEC12 in GC remain unclear.
This study found a significant correlation between COLEC12 expression and the clinicopathological features of GC. High COLEC12 expression was significantly correlated with highly advanced stages (T3-T4, pathologic stage III-IV) in GC patients, suggesting that COLEC12 expression is associated with tumor progression in GC. Therefore, knockdown and overexpression experiments specific to COLEC12 were performed to determine its role in GC progression in vitro. COLEC12 knockdown suppressed GC cell proliferation, migration, and invasion and induced GC cell apoptosis. However, COLEC12 overexpression significantly increased GC cell proliferation, migration, and invasion. Furthermore, COLEC12 overexpression inhibited antitumor drug-induced GC cell apoptosis. These results show that COLEC12 positively regulates GC cell growth in vitro.
An effective prognostic biomarker can be used to predict patient clinical outcomes and guide clinical personalized medical treatment. Recent studies have reported that SARC patients with high COLEC12 expression have a poor prognosis. Thus, we determined the value of COLEC12 expression in predicting PFS and OS in GC patients. According to our survival analysis, high COLEC12 expression in tumor tissues predicted poor PFS and OS in GC patients. Cox univariate and multivariate analyses further confirmed that high COLEC12 expression is an independent risk factor for PFS and OS in GC patients.
Many studies have demonstrated that tumor-infiltrating immune cells are involved in the progression of different malignant tumors.19–21 Ma et al found that COLEC12 was highly expressed in umbilical cord vascular endothelial cells as a transmembrane receptor, and soluble COLEC12 could partially recognize Aspergillus fumigatus through its carbohydrate-recognition domain in a Ca2+-independent manner. This activated the alternative complement pathway exclusively through association with properdin on Aspergillus fumigatus. 8 In addition, Li et al showed that COLEC12 knockdown could increase osteosarcoma inflammation in vivo and in vitro and identified TLR4 as a possible downstream target factor. 9 However, the relationship between COLEC12 and tumor-infiltrating immune cells in GC remains unclear. The results of this study revealed that, in GC, COLEC12 expression was associated with several immune infiltrating cells, including macrophages, DCs, and neutrophils.
Based on its state of activation, function, and secretion of factors, macrophages can be divided into classically activated M1 macrophages and selectively activated M2 macrophages.22–24 M2 macrophages participate in the immunosuppression, invasion, and metastasis of various cancers and are closely related to poor prognosis.25,26 Our results showed that COLEC12 expression was moderately to strongly correlated with the expression of M2 macrophage gene markers, such as CD163, VSIG4, MS4A4A, and COLEC12, but weakly correlated with the expression of M1 macrophage gene markers, such as PTGS2 and IRF5. These results suggest that COLEC12 may be involved in GC immunosuppression by regulating TAM polarization.
DCs are typical antigen-presenting cells. Immature DCs have a strong phagocytic activity, whereas mature DCs produce various cytokines and have a strong regulatory function.27,28 DCs can increase the Treg cell number and reduce CD8 + T cell cytotoxicity to promote tumor metastasis. 29 In addition, COLEC12 expression was found to be positively correlated with DC gene markers, such as HLA-DPB1, HLA-DQB1, HLA-DRA, HLA-DPA1, BDCA-1, BDCA-4, and CD11c, suggesting that COLEC12 may mediate tumor metastasis by activating DCs.
Neutrophils are key players in inflammatory cell infiltration in different cancer types and can inhibit CD8 + T cells in the antitumor immune response. 30 CD11b is involved in immunity regulation by inducing neutrophil differentiation. 31 In this study, we found that COLEC12 was significantly correlated with CD11b in GC, suggesting that COLEC12 may inhibit tumor immunity by mediating neutrophils and their correlation with CD11b. Furthermore, COLEC12 expression was also found to be positively correlated with the expression of Treg cell markers, such as FOXP3, and T cell depletion markers, such as TIM-3. FOXP3 is an important marker in Treg cells that can inhibit cytotoxic T cells from attacking tumor cells.32,33 TIM-3 is a coinhibitory receptor expressed on T cells, macrophages, and DCs, in which TIM-3 has been shown to inhibit its response when interacting with its ligand.34–36 These results further demonstrate that COLEC12 expression may play a crucial role in the activation of Treg cells and the induction of T cell depletion, which may partly explain the correlation between the poor prognosis and the increased COLEC12 expression in GC. All these results suggest that COLEC12 may suppress tumor immunity in GC.
It is important to point out that this study also has some limitations. First, data from TCGA database may vary due to the use of different treatments and experiments in different studies. To resolve this issue, we used the limma package to correct the batch effect and also included clinical samples and in vitro experiments.
Conclusion
In summary, our findings revealed that COLEC12 was highly expressed in advanced GC. High COLEC12 expression was correlated with a worse PFS and OS in GC patients. In vitro, cell line studies indicated that COLEC12 promotes GC cell proliferation, migration, and invasion and inhibits GC cell apoptosis. The bioinformatics analysis further demonstrated a stronger correlation between COLEC12 expression and several immune infiltrating cells, such as M2, DCs, neutrophils and Tregs, indicating that COLEC12 may also play a role in suppressing the tumor immune response in GC. Overall, this study identified a novel predictive marker and target for the clinical treatment of GC. However, more studies are warranted to further elucidate the underlying mechanism by which COLC12 exerts its effects on GC.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338231218163 for COLEC12 Promotes Tumor Progression and Is Correlated With Poor Prognosis in Gastric Cancer by Xiangfei Sun, Qiang Zhang, Ping Shu, Xiaohan Lin, Xiaodong Gao and Kuntang Shen in Technology in Cancer Research & Treatment
Abbreviations
- GC
gastric cancer
- COLEC12
collectin subfamily member 12
- PRM
pattern-recognition molecule
- PFS
progression-free survival
- OS
overall survival
- CI
confidence interval
- HR
hazard ratio
- STAD
stomach adenocarcinoma
- CCK-8
cell counting kit-8
- TMA
tissue microarray
- IHC
immunohistochemistry
- qRT-PCR
quantitative real-time polymerase chain reaction
- ssGSEA
single-sample gene set enrichment analysis
- TME
tumor microenvironments
- TAM
tumor-associated macrophage
- Th cell
T helper cell
- Tfh cell
T follicular helper cell
- Treg cell
T regulatory cell
- DC
dendritic cell
- NK cell
natural killer cell.
Footnotes
Authors’ Note: Ethical Approval: This retrospective study was approved by the ethics committees of Zhongshan Hospital, Fudan University. The requirement of patient consent was waived for this study due to its retrospective nature; however, all patients provided written informed consent before the collection of biological samples. Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China, (grant number 81773080).
ORCID iD: Kuntang Shen https://orcid.org/0000-0002-9600-6508
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. [DOI] [PubMed] [Google Scholar]
- 3.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi H, Enomoto A, Woods SL, et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16(5):282-295. [DOI] [PubMed] [Google Scholar]
- 5.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69-84. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Zhang P, Shao M, et al. SALL4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res. 2018;10:4459-4470. doi: 10.2147/CMAR.S177373. PubMed 30349378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtani K, Suzuki Y, Eda S, et al. The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J Biol Chem. 2001;276(47):44222-8. [DOI] [PubMed] [Google Scholar]
- 8.Ma YJ, Hein E, Munthe-Fog L, et al. Soluble collectin-12 (CL-12) is a pattern recognition molecule initiating complement activation via the alternative pathway. J Immunol. 2015;195(7):3365-3373. [DOI] [PubMed] [Google Scholar]
- 9.Li GZ, Deng JF, Qi YZ, et al. COLEC12 regulates apoptosis of osteosarcoma through toll-like receptor 4-activated inflammation. J Clin Lab Anal. 2020;34(11):e23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elola MT, Capurro MI, Barrio MM, et al. Lewis x antigen mediates adhesion of human breast carcinoma cells to activated endothelium. Possible involvement of the endothelial scavenger receptor C-type lectin. Breast Cancer Res Treat. 2007;101(2):161-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang LL, Hsu WH, Kao MC, et al. Stromal C-type lectin receptor COLEC12 integrates H. pylori, PGE2–EP2/4 axis and innate immunity in gastric diseases. Sci Rep. 2018;8(1):3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finotello F, Trajanoski Z. Quantifying tumor-infiltrating immune cells from transcriptomics data. Cancer Immunol Immunother. 2018;67(7):1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. PubMed 23323831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782-795. [DOI] [PubMed] [Google Scholar]
- 15.Danaher P, Warren S, Dennis L, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer. 2017;5:18. doi: 10.1186/s40425-017-0215-8. PubMed 28239471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan JH, Zhou H, Cooper L, et al. LAYN is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers. Front Immunol. 2019;10:6. doi: 10.3389/fimmu.2019.00006. PubMed 30761122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Lichtenberg T, Hoadley KA, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400-416.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Liu Y, Zhang S, et al. Impacts and mechanisms of metabolic reprogramming of tumor microenvironment for immunotherapy in gastric cancer. Cell Death Dis. 2022;13(4):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sas Z, Cendrowicz E, Weinhauser I, et al. Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci. 2022;23(7):3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol. 2021;32(10):1236-1244. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Chen X, Guo C, et al. Tumour-associated macrophages heterogeneity drives resistance to clinical therapy. Expert Rev Mol Med. 2022;24:e17. doi: 10.1017/erm.2022.8. PubMed 35400355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Li S, Malik I, et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature. 2023;619(7970):616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dalen FJ, van Stevendaal M, Fennemann FL, et al. Molecular repolarisation of tumour-associated macrophages. Molecules. 2019;24(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. 2022;19(6):402-421. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7-24. [DOI] [PubMed] [Google Scholar]
- 28.Adler AJ. Cancer immunology and immunotherapy: from defining basic immunology to leading the fight against cancer. Immunol Invest. 2022;51(8):2128-2132. [DOI] [PubMed] [Google Scholar]
- 29.Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol. 2019;348:123-178. doi: 10.1016/bs.ircmb.2019.07.006. PubMed 31810552. [DOI] [PubMed] [Google Scholar]
- 30.Dai XK, Ding ZX, Tan YY, et al. Neutrophils inhibit CD8(+) T cells immune response by arginase-1 signaling in patients with sepsis. World J Emerg Med. 2022;13(4):266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173-187. [DOI] [PubMed] [Google Scholar]
- 32.Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140-1146. [DOI] [PubMed] [Google Scholar]
- 34.Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1):e000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagliano O, Morrison RM, Chauvin JM, et al. Tim-3 mediates T cell trogocytosis to limit antitumor immunity. J Clin Invest. 2022;132(9):e152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Cheng S, Fan L, et al. TIM-3: an update on immunotherapy. Int Immunopharmacol. 2021;99:107933. doi: 10.1016/j.intimp.2021.107933. PubMed 34224993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338231218163 for COLEC12 Promotes Tumor Progression and Is Correlated With Poor Prognosis in Gastric Cancer by Xiangfei Sun, Qiang Zhang, Ping Shu, Xiaohan Lin, Xiaodong Gao and Kuntang Shen in Technology in Cancer Research & Treatment