Abstract
This study evaluated the histopathological changes in the gill, liver and kidney of African catfish (Clarias gariepinus) intoxicated with a sub-lethal dose of Melaleuca cajuputi leaves extract (MCLE) for 96 h. The acute toxicity test has been determined previously with a value of 96-h LC50 = 127 mg/L, hence the selection of sub-lethal ranges from 60 mg/L to 160 mg/L of MCLE. Degenerative alterations were prominent in all tested organs, particularly after exposure to a high concentration of MCLE. Gill exhibited haemorrhage, epithelial lifting, lamellar disorganisation, and necrosis after exposure to a high MCLE concentration. Alterations in the liver include congestion, hydropic degeneration, and vacuolation, whereas lesions in the kidney were pyknosis, vacuolation, hydropic degeneration, and tubular necrosis. The obtained data showed that the organs experienced severe changes proportional to the increase in MCLE concentration. In addition, fish exposed to higher concentrations than the LC50 value experienced irreversible lesions. The present study suggests that the use of MCLE below the LC50 is recommended to avoid severe alterations to organs, particularly in African catfish. This study demonstrated that the use of MCLE above the LC50 promotes severe damage to the gills, liver and kidney of African catfish. However, further investigations are needed to define the causing-mechanisms underlying these effects.
Keywords: Gelam, Catfish, Histopathology Alterations, Sub-Lethal
Abstrak
Kajian ini menilai perubahan histopatologi dalam insang, hati dan buah pinggang ikan keli Afrika (Clarias gariepinus) yang ditoksikkan dengan dos sub-lethal ekstrak daun Melaleuca cajuputi (MCLE) selama 96 jam. Ujian ketoksikan akut telah ditentukan sebelum ini dengan nilai 96-jam LC50 = 127 mg/L, oleh itu pemilihan julat sub-lethal dari 60 mg/L hingga 160 mg/L MCLE. Perubahan degeneratif adalah ketara dalam semua organ yang diuji, terutamanya selepas terdedah kepada kepekatan MCLE yang tinggi. Insang menunjukkan pendarahan, pengangkatan epitelium, kecelaruan lamellar, dan nekrosis selepas terdedah kepada kepekatan MCLE yang tinggi. Perubahan dalam hati termasuk kesesakan, degenerasi hidropik dan vakuolasi, manakala luka pada buah pinggang adalah piknosis, vakuolasi, degenerasi hidropik dan nekrosis tiub. Data yang diperolehi menunjukkan bahawa organ-organ mengalami perubahan teruk yang berkadar dengan peningkatan kepekatan MCLE. Selain itu, ikan yang terdedah kepada kepekatan yang lebih tinggi daripada nilai LC50 mengalami luka yang tidak dapat dipulihkan. Kajian ini mencadangkan bahawa penggunaan MCLE di bawah nilai LC50 disyorkan untuk mengelakkan perubahan teruk pada organ, terutamanya pada ikan keli Afrika. Kajian ini menunjukkan bahawa penggunaan MCLE di atas nilai LC50 menggalakkan kerosakan teruk pada insang, hati dan buah pinggang ikan keli Afrika. Walau bagaimanapun, penyiasatan lanjut diperlukan untuk menentukan mekanisma punca yang menjadi penyebab kepada kesan ini.
Kata kunci: Gelam, Ikan Keli, Perubahan Histopathologi, Sub-Lethal
Highlights.
The severity of gills, liver and kidney of African catfish were proportional to the increase of Melaleuca cajuputi leaves extract (MCLE) concentrations.
Degeneration alterations were notable in gills, liver and kidney of African catfish when exposed to MCLE concentration over than the LC50 value.
It was recommended that MCLE exposure be less than the LC50 value to lessen the histopathological alterations in the gills, liver and kidneys of African catfish.
INTRODUCTION
Aquaculture is one of the fastest growing sectors in the food industry, accounting for more than 20% of all animal protein exported to low-income countries worldwide (Tacon 2020). Among them, fish farming accounts for nearly 40% (54.3 million tonnes), followed by mollusks and crustaceans at 17.7 million tonnes and 9.4 million tonnes, respectively (FAO 2020). However, intensive aquaculture with high stocking capacity promotes the spread of pathogenic microorganisms, resulting in massive economic losses (Valenzuela-Gutiérrez et al. 2021). Previously, several chemical antibiotics such as formalin, trichlorfon and mebendazole were used to control this situation (Liu et al. 2021), but their overuse resulted in antibiotic accumulation, which might pose health risks to the consumer (Aisiah et al. 2020). Furthermore, long-term use of these antibiotics may result in bacterial disease resistance and a lack of efficacy in some control agents (Zhang et al. 2014). To avoid the use of chemotherapeutical treatments, fish need to improve their immunity to combat the dispersion of diseases and pathogens. The use of plant extracts as immunostimulants in aquatic organisms has been reported worldwide through improving growth and body defense mechanisms (Sahimi et al. 2022; Doan et al. 2022; Rashidian et al. 2021; Nafiqoh et al. 2020; Mohammadi et al. 2020). Therefore, the use of plant extracts as an alternative is promising as a potential strategy to improve fish immunity to combat diseases and parasites due to their advantages of being environmentally friendly and biodegradable (Liu et al. 2021).
Melaleuca cajuputi is one of the native plants that are commonly found throughout Southeast Asia and Australasia (Nagi et al. 2016). Major phytochemical compounds such as terpenes, alkaloids, phenolics and flavonoids were previously reported in M. cajuputi extract which contribute to a broad-spectrum of biological activities (Chaudhari et al. 2022; Sahimi et al. 2022; Septiana et al. 2020; Bua et al. 2018; Chan & Haron 2016; Al-Abd et al. 2015). Some of them demonstrated their toxicity through acute toxicity tests (Jayakumar et al. 2020; Noor et al. 2021; Bakar 2020; Roszaini et al. 2013), but not all of the activity has been effectively characterised in vivo to determine their safety effect at a sub-lethal dose. There are few reports of histopathological changes following the use of plant extracts on aquatic organisms (Meneses et al. 2020; Karataş et al. 2020; Meneses et al. 2018; Shivappa et al. 2015), but there is a paucity in the M. cajuputi plant extract. Therefore, this study was performed to determine the histopathological changes occur in the gills, liver, and kidney of African catfish at sub-lethal doses. Earlier, an acute toxicity study of Melaleuca cajuputi leaves extract (MCLE) had been done on the catfish, Clarias gariepinus, which gave a 96-hour LC50 of 127 mg/L (Nagi et al. 2016). The findings may be useful in determining the safety of using MCLE as a baseline for fish, particularly African catfish, as therapeutics.
MATERIALS AND METHODS
Collection and Identification of M. cajuputi Powell
Fresh Melaleuca cajuputi leaves were collected in Marang, Terengganu (5.4932°N, 102.9320°E) and sent to botanist for taxonomy identification under the voucher number of MFI 0244/22.
Preparation of M. cajuputi leaves Extract (MCLE) and Treatment Solution
Leaves were washed under running tap water to remove any soil debris and dried at room temperature (27 ± 2°C) for 14 days. Dried leaves were ground and weighed for 10 g before soaking in 80% methanol for 24 h with shaking (27°C, 200 rpm). The mixture was then filtered using filter paper (Whatman, UK) and the filtrate was further concentrated using a rotary evaporator (Buchi Rotavapor-R, Fisherscience, UK) at 38°C until the gummy-like crude extract was formed. The crude extract was freeze-dried and the powder form was stored at −20°C until further use.
Phytochemical Compound Identification Using Gas Chromatography-Mass Spectra (GC-MS) Analysis
GC-MS analysis was performed according to Al-Abd et al. (2015), with slight modifications. Briefly, 10 mg of MCLE sample was sonicated for 15 min in 2.5 mL of dichloromethane at 40°C in a sealed vial. Then, 1 mL of the mixture was filtered through a 0.20 μM nylon filter, and 1 μL of the filtrate was injected splitless into the GC-MS system (Hawlett-Packard autosampler 6890 GC, Agilent, USA), equipped with a capillary column (diameter: 30 m × 0.25 mm; thickness: 0.24 μM). The column initial temperature was initially set up at 40°C for 3 min, and the final temperature was at 280°C for 3 min. Helium was used as a carrier gas at 1 mL/min flow rate. The ion source temperature and injector were both maintained at 250°C. The mass spectrometer was scanned over the range of 28–400 m/z amu at 1 scan−1, with an ionising voltage of 70 eV. The spectra that came up were then compared with the known compounds from the NIST database library based on their retention time (RT) index of primary and secondary metabolites.
Fish Acclimatisation
Clarias gariepinus with a mean body weight and total length of 20.0 ± 3.0 g and 15.0 ± 2.0 cm, respectively, were obtained from the hatchery at the Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (UMT). Fish were placed in a tank containing 500 L of non-chlorinated water and acclimatised for two weeks under laboratory conditions. The fish were fed daily with commercial fish pellets at 3% of their body weight. The water quality parameters, including temperature, pH and dissolved oxygen, were measured using multiparameter equipment (YSI 556 MPS, USA) and maintained at 26°C–28°C, 5.50–6.25 and >5 mg/L, respectively. Approximately 80%–90% of the water was changed daily to keep the quality.
Experimental Procedure
The acute toxicity and behavioral studies have been performed previously by Nagi et al. (2016), which indicated the 96-hour LC50 value of MCLE to be at 127 mg/L. In this present study, a sub-lethal toxicity test was performed to determine the histological changes on gills, liver and kidney of African catfish after exposure to MCLE for 96 h, as according to Correia et al. (2020), with slight modifications. Briefly, six MCLE concentration were used in this study, (i.e., 0.0, 60, 80, 100, 120, 140 and 160 mg/L), where 0.0 mg/L was served as control. Healthy acclimatised fish were randomly distributed in 18 aquaria (50 L) containing MCLE solutions, with 5 fish per treatment, and in triplicates (5 fish × 6 MCLE concentrations × 3 replicates). Water parameters including temperature, pH and dissolved oxygen were controlled at 26°C–28°C, 5.5–6.25 and >5 mg/L, respectively using YSI multi-parameter probe (YSI Inc., USA). No feeding was performed during the experiment.
Histological Assessment of Gills, Liver and Kidney of African Catfish
At the end of experiment, fish were euthanised by rapid cooling (Rodrigues et al. 2019), decapitated, and the gills, liver and kidney were carefully removed. Thereafter, the organ tissues were fixed in 10% formalin solution, followed by dehydration through six grades of alcohol separately, sectioned using a microtome (Leica, Germany) sectioning, and stained with H&E. Finally, the mounted slides were examined under a light microscope (Leica, Germany) to observe any histological changes in the tissues. Histopathological changes observed in tissue were captured using a digital camera attached with software (Leica Application Suite V4.0, Leica, Germany) at various magnifications. The histopathological alterations were evaluated using quantitative histological assessment as adopted by Bernet et al. (1999), and Nero et al. (2006), with some modifications. Each organ were classified into four reaction patterns (rp): circulatory, degeneration, inflammatory and structural. The score values from 1 to 6 (a) were assigned based on the percentage of each alteration, whereas the importance factors (w) ranging from 1 to 3 indicate how the alterations might affect the fish health. The calculation for each category of alterations and the total pathological index were defined as:
where org = organ, cat = category, alt = alteration, a = score and w = importance factor.
Statistical Analysis
Data normalities were checked prior to statistical analysis using normal probability plots. The statistical differences between the control and MCLE-exposed fish were determined using one-way analysis of variance (ANOVA), and Tukey pairwise test at p < 0.05 to indicate the significant difference relative to the control group (Genstat Software ver. 12.1).
RESULTS
Determination of Phytochemical Compounds in MCLE using GC-MS Analysis
A total of 37 compounds were identified in MCLE as shown in Table 1. There are four major compounds were detected including 1H-imidazole, 4,5-diphenyl- (18.60%), followed by 7-methyl-6,8-bis(methylthio)pyrrolo[1,2]pyrazine (15.43%), 2-isopropyl-10-methylphenanthrene (13.50%) and 9-carboxaldehyde-10-methylanthracene (11.13%) within 30 min of retention time (Fig. 1).
Table 1.
Phytochemical compounds in MCLE identified by GC-MS analysis.
| No. | RT (min) | Name of the compound | Molecular formula | Molecular weight | Peak area (%) |
|---|---|---|---|---|---|
| 1 | 5.598 | 1-(1-methylethyl)-4-methyl-3-Cyclohexen-1-ol, | C10H18O | 154.2493 | 0.25 |
| 2 | 5.655 | Benzene, 1-methyl-4-(1-methylethenyl)- | C10H12 | 132.2023 | 0.38 |
| 3 | 5.724 | 3-Cyclohexene-1-methanol,.alpha.,.alpha.,4-trimethyl-, (S)- | C10H18O | 154.25 | 0.35 |
| 4 | 8.957 | Naphthalene, 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl) | C15H24 | 204.3511 | 0.19 |
| 5 | 9.048 | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylethenyl) | C15H24 | 204.3511 | 0.26 |
| 6 | 9.2941 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | C15H24 | 204.3511 | 0.22 |
| 7 | 9.569 | Cyclohexanemethanol, 4-ethenyl-.alpha.,.alpha.,4-trimethyl-3-(1-methylethenyl) | C15H26O | 222.3663 | 0.52 |
| 8 | 9.912 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-, [1ar-(1a.alpha.,4a.alpha.,7.beta.,7a.beta.,7b.alpha.)]- | C15H24O | 220.3505 | 2.26 |
| 9 | 9.981 | Caryophyllene oxide | C15H24O | 220.35 | 2.07 |
| 10 | 10.084 | Guaiol | C15H26O | 222.37 | 3.32 |
| 11 | 10.227 | 7-Methyl-6,8-bis(methylthio)pyrrolo[1,2]pyrazine | C10H12N2S2 | 224.34 | 15.43 |
| 12 | 10.398 | Cycloisolongifolene, 8,9-dehydro | C15H22 | 202.3352 | 1.66 |
| 13 | 10.438 | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, (2R-cis) | C15H26O | 222.3663 | 1.96 |
| 14 | 10.496 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | C15H24O | 220.3505 | 1.14 |
| 15 | 10.644 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | C15H26O | 222.3663 | 7.25 |
| 16 | 10.776 | 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl-, [1ar-(1a.alpha.,4.beta.,4a.beta.,7.alpha.,7a.beta.,7b.alpha.)]- | C15H26O | 222.3663 | 1.42 |
| 17 | 10.810 | Humulen-(v1) | C15H24 | 204.3510 | 0.97 |
| 18 | 10.956 | 7R,8R-8-Hydroxy-4-isopropylidene-7-methylbicyclo[5.3.1]undec-1-ene | C15H24O | 0.70 | |
| 19 | 11.148 | 2-Acetyl-5-chloro-3-methylbenzo(b)thiophene | C11H9ClOS | 224.7066 | 5.45 |
| 20 | 11.280 | Bicyclo[4.3.0]nonane, 7-methylene-2,4,4-trimethyl-2-vinyl- | C15H24 | 204.3510 | 0.81 |
| 21 | 11.308 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | C15H24 | 204.3510 | 0.77 |
| 22 | 11.743 | 1-(3-Chloromethyl-2,4,6-trimethylphenyl)ethanone | C12H15ClO | 210.6999 | 1.43 |
| 23 | 11.783 | p-isopropylphenetole | C11H16O | 164.2441 | 0.21 |
| 24 | 12.064 | 2’,3’,4’,5’,6’-pentafluoroacetophenone | C8H3F5O | 210.1 | 0.43 |
| 25 | 12.149 | 2-naphthalenemethanol, 1,2,3,4,4a,5,6,8a-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | C15H26O | 222.3663 | 0.57 |
| 26 | 12.727 | 4H-1-benzopyran-4-one, 5-hydroxy-7-methoxy-2-methyl- | C11H10O4 | 206.19 | 0.59 |
| 27 | 12.956 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.4507 | 0.29 |
| 28 | 13.299 | Hexadecanoic acid | C16H32O2 | 256.4241 | 0.42 |
| 29 | 13.591 | 9-carboxaldehyde-10-methylanthracene | C16H12O | 220.27 | 11.13 |
| 30 | 13.643 | 9H-fluoren-9-ol, 9-butyl- | C17H18O | 238.3242 | 3.04 |
| 31 | 13.780 | 1H-imidazole, 4,5-diphenyl- | C15H12N2 | 220.2692 | 18.60 |
| 32 | 13.940 | 2-isopropyl-10-methylphenanthrene | C18H18 | 234.34 | 13.50 |
| 33 | 14.495 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 0.50 | ||
| 34 | 14.695 | 9,12,15-octadecatrien-1-ol | C18H32O | 264.4461 | 0.42 |
| 35 | 17.677 | 1H-indole, 5-methyl-2-phenyl- | C15H13N | 207.2704 | 022 |
| 36 | 17.763 | 5-methyl-2-phenylindolizine | 0.27 | ||
| 37 | 26.088 | Stigmasterol, 22,23-dihydro- | C29H50O | 414.7067 | 1.02 |
Figure 1.

Total ion chromatogram of MCLE by GC-MS analysis.
Histopathological Assessment
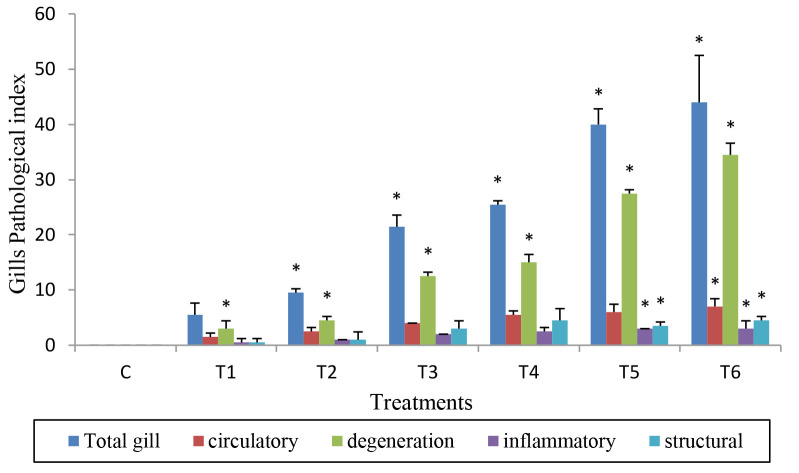
Overall, the gills, liver and kidney of African catfish showed significant pathological alterations when exposed to a higher concentration of MCLE (p < 0.05) than the control. All organs showed pathological indices associated with the elevation of MCLE concentrations, particularly starting at 120 mg/L to 160 mg/L. Degenerative alterations predominated (p < 0.05) in all the tested organs of MCLE-treated fish compared to control (Figs. 2A, 2B and 2C). Concerning to gills and liver, necrosis was recorded at higher MCLE concentrations (120 mg/L to 160 mg/L), whereas started later at 140 mg/L to 160 mg/L in the kidney. However, nuclear alterations (e.g., pyknosis) and hydropic degeneration were already observed in the kidney of fish exposed to 120 mg/L. In terms of circulatory changes, sinusoids congested with red blood cells and cytoplasmatic vacuolation were observed in the liver at lower exposures of MCLE. Among the reported circulatory changes, haemorrhage was the most detected in fish kidney, even increasing the lowest exposure of MCLE concentration.
Figure 2.
Pathological indices of C. gariepinus (A) gills, (B) liver and (C) kidney at different concentration of MCLE. C = Control; T1 = 60 mg/L; T2 = 80 mg/L; T3 = 100 mg/L; T4 = 120 mg/L; T5 = 140 mg/L and T6 = 160 mg/L. Asterisk (*) have significant different at p < 0.05.
No histological changes were observed in the gills, liver and kidney of non-treated fish (Fig. 3A). However, several changes were observed in MCLE-treated fish with the severity increasing as the plant extract concentration increased. In the 60 mg/L MCLE (Fig. 3B), a minor degree of oedema, hydropic degeneration of the epithelial cells, and lamella fusion were observed. High tissue alteration was observed in 80 mg/L MCLE (Fig. 3C), whereas lesion was observed in 100 mg/L MCLE, including epithelial lifting, congestion, lamellar epithelial cell degeneration, and oedema (Fig. 3D). Severe lesions including haemorrhage, lamella fusion, oedema, and necrosis were observed in 120 mg/L MCLE (Fig. 3E). Severe degenerative and necrotic changes in gill epithelia were detected in gills exposed to 140 mg/L and 160 mg/L MCLE (Figs. 3F, 3G), with huge lamellar disorganisation and aneurisms found only in 160 mg/L MCLE (Fig. 3G).
Figure 3.







(A) Normal gills of C. gariepinus (0 mg/L MCLE); (B) 60 mg/L of MCLE with 1. Oedema, 2. Hydropic degeneration of the epithelial cells; (C) 80 mg/L MCLE with 1. Epithelial lifting, 2. Vines congestion, 3. Oedema, 4. Hydropic degeneration of the epithelial cells; (D) 100 mg/L MCLE with 1. Epithelial lifting, 2. Vines congestion, 3. Epithelial cells degeneration, 4. Oedema; (E) 120 mg/L MCLE with 1. Haemorrhage, 2. Lamellar epithelial cells degeneration, 3. Oedema, 4. Necrosis; (F) 140 mg/L MCLE with 1. Epithelial lifting of secondary lamellae, 2. Necrosis, 3. Hydropic degeneration of the epithelial cells; (G) 160 mg/L MCLE with 1. Lamellar disorganisation, 2. Haemorrhage, 3. Necrosis, 4. Oedema. H&E stain, 400× total magnifications.
No lesions were observed in non-treated fish liver (Fig. 4A). Meanwhile, alterations were observed in liver tissues at 60 mg/L MCLE, including congestion of sinusoids and vacuolation (Fig. 4B). A slight changes like plasma alteration, degeneration, and leukocyte infiltration in fish exposed to 80 mg/L MCLE (Fig. 4C). More alteration was found in higher MCLE concentration such as congested of central vein with poor hepatic cord structure (Fig. 4D) and hepatocytes focal necrosis (Fig. 4E), and even more severe in the higher MCLE concentration (Fig. 4F). For the kidney, a normal appearance was observed in the untreated kidney tissues (Fig. 5A). However, a slight change was observed with the increased plant concentration (Fig. 5B), including tubular congestion with red blood cells and hydropic degeneration (Fig. 5C), vacuolation, hydropic degeneration of tubular cells (Fig. 5D), and severe hydropic degeneration of tubular cells, haemorrhage and tubular necrosis (Figs. 5E and 5F).
Figure 4.







(A) Normal liver of C. gariepinus (0 mg/L MCLE) with 1. Hepatocytes, 2. Hepatic portal vein, 3. Hepatic artery, 4. Bile duct; (B) 60 mg/L MCLE with 1. Sinusoid congested with red blood cells, 2. Cytoplasmatic vacuolation; (C) 80 mg/L MCLE with 1. Central vein congested with red blood cells, 2. Infiltration of leukocytes, 3. Vacuolation, 4. Cloudy degeneration, 5. Aggregation of inflammatory cell between hepatocytes; (D) 100 mg/L MCLE with 1. Structure alteration; (E) 120 mg/L MCLE with 1. Focal necrosis of hepatocytes, 2. Hydropic degeneration; (F) 140 mg/L MCLE with 1. Vacuolation, 2. Degeneration, 3. Necrosis, 4. Increase in sinusoidal spaces and congested with red blood cells; (G) 160 mg/L MCLE with severely necrotic liver tissues, vacuolation of hepatocytes, hydropic degeneration of hepatocytes and poor hepatic cord structural. H&E stain, 400× total magnifications.
Figure 5.




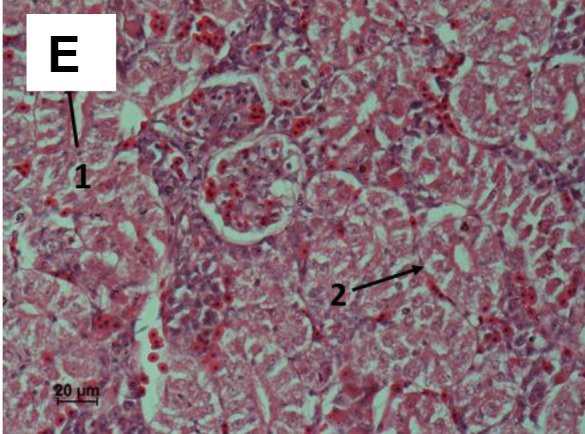


(A) Normal kidney of C. gariepinus (0 mg/L MCLE) with 1. Glomerulus, 2. Bowman’s space well defined, 3. Proximal tubule, 4. Distal tubule, 5 Bowman’s capsule, 6. Hematopoietic tissue; (B) 60 mg/L MCLE with 1. Haemorrhage; (C) 80 mg/L MCLE with 1. Haemorrhage, 2. Tubular degeneration; (D) 100 mg/L MCLE with 1. Shrinkage of glomerulus, 2. Vacuolation and hydropic degeneration; (E) 120 mg/L MCLE with 1. Nuclear alterations, 2. Vacuolation and hydropic degeneration; (F) 140 mg/L MCLE with 1. Haemorrhage, 2. Tubular necrosis; (G) 160 mg/L MCLE with 1. Severely necrotic kidney glomerulus, 2. Vacuolation, 3. Tubular degeneration, 4. Dilatation of glomerulus capillaries, 5. Hematopoietic tissue with red blood cells and poor tubular structural. H&E stain, 400× total magnifications.
DISCUSSION
The histopathological evaluation of tissue and cells caused by toxicants is crucial to determining the health condition of organisms. In this present study, MCLE was exposed for 96 h to African catfish at sub-lethal doses, and the histological alterations in the gills, liver, and kidney were observed. In the GC-MS analysis, 37 compounds were detected in MCLE, with terpenes and flavonoids being the major compounds. Terpenes such as caryophyllene oxide (2.07%), 1H-imidazole, 4,5-diphenyl-18.60%, 2-isopropyl-10-methylphenanthrene (13.50%), and stigmasterol (1.02%) are among the higher peaks found in MCLE, which have also been reported previously (Noor et al. 2021; Białoń et al. 2019; Ukit et al. 2019), however, with a slight difference in quantity, which might be influenced by several geographical factors including method of extraction, harvesting period, part of the plant used and more (Kumari et al. 2014). These compounds may act synergistically or in a single compound to serve a variety of biological properties such as antibacterial (González et al. 2021; Moghrovyan et al. 2019), anti-inflammatory (Al-Khayri et al. 2022; Prado-Audelo et al. 2021), antioxidant (Ivanović et al. 2020; Amri & Hossain 2018), antifungal (Salomé-Abarca et al. 2020; de Lima et al. 2022), and many more.
An acute toxicity test of MCLE on African catfish has been conducted previously by Nagi et al. (2016), which resulted in a 96-hour LC50 of 127 mg/L. Following that, a sub-lethal toxicity test was performed in this current study to determine the histopathological effects on the gills, liver, and kidney of African catfish during the exposed time. Gills are a good indicator of water quality because of their large surface area and contacts with the external environment (Strzyzewska et al. 2016). The harmful chemical and biological substances in the water surroundings may influence the gills’ morphological changes and impairment of their functions. The results of this study revealed that histopathological changes such as epithelial lifting, oedema and hydropic degeneration were observed in fish gills exposed to lower concentrations of MCLE, but the effects increased proportionally with MCLE concentrations, such as lamellar disorganisation and necrosis. It can be speculated that these significant alterations may be attributed to the high content of phytochemical compounds in MCLE. Although mucous secretion aids in the prevention of toxicants passing to the gill epithelium, these consequences may disturb the gas exchange process and reduce respiration (Nero et al. 2006). Similar findings were reported by Pal et al. (2012) after exposure of chlorpyrifos to common carp, Cyprinus carpio. The common alterations observed in all concentrations were epithelial lifting and hydropic degeneration, which increased proportionally with toxicant concentrations. Similar alterations were found in Sparus aurata following acute and chronic exposure to erythromycin and oxytetracycline (Rodrigues et al. 2019). These alterations may be induced by severe oedema, leading to a partial or complete fusion of the gill filament (Yancheva et al. 2016).
The liver is a primary organ for detoxification of toxicants, storage of nutrients, metabolism, and synthesis of enzymes; it therefore becomes highly vulnerable to toxicants (Rodrigues et al. 2019). High exposure to MCLE resulted in focal necrosis, vacuolation, increase in sinusoidal spaces, and poor hepatic cord structure. Vacuolation can happen due to biochemical disturbances leading to slightly glycogen flocculent and single/multiple lipid accumulation in hepatocyte cytoplasms (Yancheva et al. 2016). Similar observations were found in fish exposed to toxicants (Nunes et al. 2015; Rodrigues et al. 2017). Liver necrosis is primarily induced by oxidative stress generated by toxicants (Verma et al. 2007) and was only found in higher exposures of MCLE. It may lead to irreversible liver damage like pyknotic nucleus with a long period of exposure due to nucleus hyperactivity (Pal et al. 2012).
A significant pathological index in the kidney was mainly attributed to degenerative alterations such as tubular degeneration coupled with nuclear alterations, which indicates that the exposure of MCLE at 100 mg/L to 160 mg/L was toxic to fish even at a shorter time. Tubule degeneration and necrosis were also reported by Correia et al. (2020) after acute effects of cerium dioxide nanoparticles on Oncorhynchus mykiss. In addition, a significant circulatory disturbance was observed in the kidney, including haemorrhage and shrinkage of the glomerulus, suggesting that MCLE affects cell permeability and causes disturbance to fluid transport in and out of cells (Hadi & Alwan 2012). Finding was contradicted with Shivappa et al. (2015) where they discovered no severe changes was observed in liver and heart tissues of goldfish and clownfish after exposure to M. cajuputi extract product, Melafix.
CONCLUSION
During short-term application, the exposure of MCLE at acute levels showed several mild and moderate histological alterations in gills, liver, and kidney of African catfish, possibly due to effect of phytochemical compounds contain in extract. Additionally, the effects were dose-dependent, and using high MCLE concentrations, especially above the LC50 value (i.e., 120 mg/L to 160 mg/L), severely damages the organs’ tissues and structural integrity, potentially leading to organ malfunction. To ascertain their effectiveness, more research in other aquatic animal species may prove useful, as different animals may behave differently when exposed to plant extracts.
ACKNOWLEDGEMENTS
The research was supported by the Ministry of Higher Education, Malaysia, under the Exploratory Research Grant Scheme (ERGS) (Vot. No. 55053), and the Ministry of Higher Education, Malaysia, under the Higher Institute Centre of Excellence (HICoE) programme (Vot. No. 63933 and 56051).
Footnotes
AUTHORS CONTRIBUTIONS: Marina Hassan: The leader who initiated the project, monitored the authors during the experiment and data analysis, and was greatly contributed in writing the manuscript.
Anur Abdalah Nagi Melad: Conducted all the experiments, data analysis and writing the manuscript.
Mohd Ihwan Zakariah: Data analysis and writing the manuscript.
Nor Asma Husna Yusoff: Data analysis and writing the manuscript.
REFERENCES
- Aisiah S, Prajitno A, Maftuch M, Yuniarti A. Effect of Nauclea subdita (Korth.) Steud. leaf extract on haematological and histopathological changes in liver and kidney of striped catfish infected by Aeromonas hydrophila. Veterinary World. 2020;13(1):47–53. doi: 10.14202/vetworld.2020.47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abd N, Nor ZM, Mansor M, Azhar F, Hasan MS, Kassim M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complementary and Alternative Medicine. 2015;15:385. doi: 10.1186/s12906-015-0914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: A review. Molecules. 2022;27:2901. doi: 10.3390/molecules27092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri FS, Hossain MA. Comparison of total phenols, flavonoids and antioxidant potential of local and imported ripe bananas. Egyptian Journal of Basic and Applied Sciences. 2018;5(4):245–251. doi: 10.1016/j.ejbas.2018.09.002. [DOI] [Google Scholar]
- Bakar AA. Bioactivity evaluation of Melaleuca cajuputi (Myrtales: Myrtaceae) crude extracts against Aedes mosquito. Tropical Agricultural Science. 2020;43(3):303–313. [Google Scholar]
- Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases. 1999;22(1):25–34. [Google Scholar]
- Białoń M, Krzyśko-Łupicka T, Nowakowska-Bogdan E, Wieczorek PP. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules. 2019;24(18):3270. doi: 10.3390/molecules24183270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua A, Malicotti P, Donadu MG, Usai D, Le LS, Tran TTT, Ngo VQT, Marchetti M, Usai M, Cappuccinelli P, Zanetti S. ‘In vitro’ activity of Melaleuca cajuputi against mycobacterial species. Natural Product Research. 2018;34(10):1494–1497. doi: 10.1080/14786419.2018.1509335. [DOI] [PubMed] [Google Scholar]
- Chan BK, Haron H. Insights into putative health implications of Gelam (Melaleuca cajuputi) honey: Evidence from in-vivo and in-vitro studies. Medical Sciences. 2016;4(1):3. doi: 10.3390/medsci4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari AK, Singh VK, Das S, Kujur A, Deepika, Dubey NK. Unveiling the cellular and molecular mode of action of Melaleuca cajuputi Powell. essential oil against aflatoxigenic strains of Aspergillus flavu isolated from stored maize samples. Food Control. 2022;138:109000. doi: 10.1016/j.foodcont.2022.109000. [DOI] [Google Scholar]
- Correia AT, Rodrigues S, Ferreira-Martins D, Nunes AC, Ribeiro MI, Antunes SC. Multi-biomarker approach to assess the acute effects of cerium dioxide nanoparticles in gills, liver and kidney of Oncorhynchus mykiss. Comparative Biochemistry and Physiology, Part C. 2020;238:108842. doi: 10.1016/j.cbpc.2020.108842. [DOI] [PubMed] [Google Scholar]
- de Lima LF, Andrade-Pinheiro JC, Freitas MA, da Silva AI, Fonseca VJA, da Silva TG, da Silva JCP, de Lima RH, Sales DL, Neves RP, et al. Anti-candida properties of Gossypium hirsutum L.: Enhancement of fungal growth, biofilm production and antifungal resistance. Pharmaceutics. 2022;14:698. doi: 10.3390/pharmaceutics14040698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan HV, Lumsangkul C, Sringarm K, Hoseinifar SH, Dawood MAO, Haron EE, Harikrishnan R, Jaturasitha S, Paolucci M. Impacts of Amla (Phyllanthus emblica) fruit extract on growth, skin mucosal and serum immunities, and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquaculture Reports. 2022;22:100953. doi: 10.1016/j.aqrep.2021.100953. [DOI] [Google Scholar]
- FAO. The state of the world fisheries and aquaculture 2020: Sustainability in action. Food and Agriculture Organization of the United Nations; 2020. [Google Scholar]
- González A, Casado J, Lanas A. Fighting the antibiotic crisis: Flavonoids as promising antibacterial drugs against Helicobacter pylori infection. Frontiers in Cellular and Infection Microbiology. 2021;11:709749. doi: 10.3389/fcimb.2021.709749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi AA, Alwan SF. Histological changes in gills, liver and kidney of freshwater fish, Tilapia zillii, exposed to aluminium. International Journal of Pharmaceutical Life Sciences. 2012;3(11):2071–2081. [Google Scholar]
- Ivanović S, Avramović N, Dojćinović B, Trifunović S, Novaković M, Tešević V, Mandić B. Chemical composition, total phenols and flavonoids contents and antioxidant activity as nutritive potential of roasted hazelnut skins (Corylus avellana L.) Foods. 2020;9:430. doi: 10.3390/foods9040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar M, Ramachandran M, Krisnaveni T, Nattudurai G. Toxicity and biochemical effects of essential oils of Anethum graveolens L. and Melaleuca cajuputi Powell against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) International Journal of Tropical Insect Science. 2020;41:945–951. doi: 10.1007/s42690-020-00359-6. [DOI] [Google Scholar]
- Karataş T, Korkmaz F, Karataş A, Yildirim S. Effects of rosemary (Rosmarinus officinalis) extract on growth, blood biochemistry, immunity, antioxidant, digestive enzymes and liver histopathology of rainbow trout, Oncorhynchus mykiss. Aquaculture Research. 2020;26:1533–1541. doi: 10.1111/anu.13100. [DOI] [Google Scholar]
- Kumari S, Pundhir S, Priya P, Jeena G, Punetha A, Chawla K, Jafaree ZF, Mondal S, Yadav G. EssOilDB: A database of essential oils reflecting terpene composition and variability in the plant kingdom. Database. 2014;2014:1–12. doi: 10.1093/database/bau120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GL, Liu L, Hu Y, Wang GX. Evaluation of the antiparasitic activity of coumarin derivatives against Dactylogyrus intermedius in goldfish (Carassius auratus) Aquaculture. 2021;533:736069. doi: 10.1016/j.aquaculture.2020.736069. [DOI] [Google Scholar]
- Meneses JO, Cunha FdS, Dias JAR, da Cunha AFS, dos Santos FJ, Sousa NdC, do Couto MVdS, Paixão PEG, Abe HA, Lima BdS, et al. Acute toxicity of hot aqueous extract from leaves of the Terminalia catappa in juvenile fish Colossoma macropomum. Aquaculture International. 2020;28:2379–2396. doi: 10.1007/s10499-020-00596-z. [DOI] [Google Scholar]
- Meneses JO, do Couto MVS, Sousa NC, Cunha FS, Abe HA, Ramos FM, Chagas EC, Chaves FCM, Martins ML, Maria AN, Carneiro PCF, Fujimoto RY. Efficacy of Ocimum gratissimum essential oil against the monogenean Cichlidogyrus tilapiae gill parasite of Nile tilapia. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2018;70(2):497–504. doi: 10.1590/1678-4162-9667. [DOI] [Google Scholar]
- Moghrovyan A, Sahakyan N, Babayan A, Chichoyan N, Petrosyan M, Trchounian A. Essential oil and ethanol extract of oregano (Origanum vulgare L.) from armenian flora as a natural source of terpenes, flavonoids and other phytochemicals with antiradical, antioxidant, metal chelating, tyrosinase inhibitory and antibacterial activity. Current Pharmaceutical Design. 2019;25:1809–1816. doi: 10.2174/1381612825666190702095612. [DOI] [PubMed] [Google Scholar]
- Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Doan HV. Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio) Fish and Shellfish Immunology. 2021;99:267–273. doi: 10.1016/j.fsi.2020.01.032. [DOI] [PubMed] [Google Scholar]
- Nafiqoh N, Sukenda S, Zairin M, Alimuddin A, Lusiastuti A, Sarter S, Caruso D, Avarre JC. Antimicrobial properties against Aeromonas hydrophila and immunostimulant effect on Clarias gariepinus of Piper betle, Psidium guajava, and Tithonia diversifolia plants. Aquaculture International. 2020;28:1–13. doi: 10.1007/s10499-019-00439-6. [DOI] [Google Scholar]
- Nagi AA, Abdullah MI, Jasmani S, Zakariah MI, Karim NU, Hassan M. Acute toxicity and behavioural responses of African catfish, Clarias gariepinus to a methanolic extracts of Melaleuca cajuputi leaves. Research Journal of Fisheries and Hydrobiology. 2016;11(7):1–6. [Google Scholar]
- Nero V, Farwell A, Lister A, Van Der Kraak G, Lee LEJ, Van Meer T, Dixon DG. Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicology and Environmental Safety. 2006;63(3):365–377. doi: 10.1016/j.ecoenv.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Noor AAM, Yusuf SM, Abdul Wahab WNAW, Adam MFIC, Sul’ain MD. Evaluation on composition, antioxidant and toxicity of Melaleuca cajuputi leaves. Advances in Traditional Medicine. 2021;21:693–699. doi: 10.1007/s13596-020-00479-x. [DOI] [Google Scholar]
- Nunes B, Antunes SC, Gomes R, Campos JC, Braga MR, Ramos AS, Correia AT. Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Archives of Environmental Contamination and Toxicology. 2015;68:371–381. doi: 10.1007/s00244-014-0101-z. [DOI] [PubMed] [Google Scholar]
- Pal S, Kokushi E, Koyama J, Uno S, Ghosh AR. Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes. 2012;47(3):180–195. doi: 10.1080/03601234.2012.632285. [DOI] [PubMed] [Google Scholar]
- Prado-Audelo MLD, Cortés H, Caballero-Florán IH, González-Torres M, Escutia-Guadarrama L, Bernal-Chávez SA, Giraldo-Gomez DM, Magaña JJ, Leyva-Gómez G. Therapeutic applications of terpenes on inflammatory diseases. Frontiers in Pharmacology. 2021;12:704197. doi: 10.3389/fphar.2021.704197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian G, Boldaji JT, Rainis S, Prokić MD, Faggio C. Oregano (Origanum vulgare) extract enhances zebrafish (Danio rerio) growth performance, serum and mucus innate immune responses and resistance against Aeromonas hydrophila challenge. Animals. 2021;11(2):299. doi: 10.3390/ani11020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues S, Antunes SC, Nunes Band Correia AT. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environmental Science and Pollution Research. 2019;26:15481–15495. doi: 10.1007/s11356-019-04954-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Antunes SC, Nunes Band Correia AT. Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environmental Toxicology and Pharmacology. 2017;53:164–176. doi: 10.1016/j.etap.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Roszaini K, Nor Azah MA, Mailina J, Zaini S, Faridz ZM. Toxicity and antitermite activity of the essential oils from Cinnamomum camphora, Cymbopogon nardus, Melaleuca cajuputi and Dipterocarpus sp. against Coptotermes curvignathus K. Wood Science Technology. 2013;47:1273–1284. doi: 10.1007/s00226-013-0576-1. [DOI] [Google Scholar]
- Sahimi MBMK, Nagi AM, Hamdan NA, Zakariah MI, Yusoff NAH, Tosin OV, Hassan M. Cajeput Melaleuca cajuputi extract supplementation in diets of Macrobrachium rosenbergii: insight on the growth, immunological responses and resistance against Aeromonas hydrophila. Aquaculture Research. 2022;53(9):3441–3452. doi: 10.1111/are.15851. [DOI] [Google Scholar]
- Salome-Abarcaa LF, Mandronea M, Sannac C, Poli F, Hondel C, Klinkhamere PGL, Choi YH. Metabolic variation in Cistus monspeliensis L. ecotypes correlated to their plant-fungal interactions. Phytochemistry. 2020;176:112402. doi: 10.1016/j.phytochem.2020.112402. [DOI] [PubMed] [Google Scholar]
- Septiana S, Yuliana ND, Bachtiar BM, Putri SP, Fukusaki E, Laviña WA, Wijaya CH. Metabolomics approach for determining potential metabolites correlated with sensory attributes of Melaleuca cajuputi essential oil, a promising flavor ingredient. Journal of Bioscience and Bioengineering. 2020;129(5):581–587. doi: 10.1016/j.jbiosc.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Shivappa RB, Christian LS, Noga EJ, Law JM, Lewbart GA. Laboratory evaluation of safety and efficacy for Melafix (Melaleuca cajuputi extract) Journal of Exotic Pet Medicine. 2015;24:188–192. doi: 10.1053/j.jepm.2015.04.020. [DOI] [Google Scholar]
- Strzyzewska E, Szarek J, Babinska I. Morphologic evaluation of the gills as a tool in the diagnostics of pathological conditions in fish and pollution in the aquatic environment: A review. Veterinarni Medicina. 2016;61:123–132. doi: 10.17221/8763-VETMED. [DOI] [Google Scholar]
- Tacon AGJ. Trends in global aquaculture and aquafeed production: 2000–2017. Reviews in Fish Science and Aquaculture. 2020;28:43–56. doi: 10.1080/23308249.2019.1649634. [DOI] [Google Scholar]
- Ukit U, Widiana A, Rahmawati E, Hasby RM. Antibacterial activities test of Cajuput leaf waste extract (Melaleuca cajuputi Powell) on pathogenic bacteria. Journal of Physics: Conference Series. 2019;1402(3):033030. doi: 10.1088/1742-6596/1402/3/033030. [DOI] [Google Scholar]
- Valenzuela-Gutiérrez R, Lago-Lestón A, Vargas-Albores F, Cicala F, Martinez-Porchas M. Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiology and Biochemistry. 2021;47:1179–1198. doi: 10.1007/s10695-021-00952-7. [DOI] [PubMed] [Google Scholar]
- Verma RS, Mehta A, Srivastava N. In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pesticides Biochemistry and Physiology. 2007;88(2):191–196. [Google Scholar]
- Yancheva VS, Stoyanova SG, Valcheva IG, Georgieva ES. Histological response of fish gills to metal pollution: common carp, Cyprinus carpio L., and common rudd, Scardinius erythrophthalmus L., from Topolnitsa Reservoir, Bulgaria. Acta Zoologica Bulgarica. 2016;68(1):103–109. [Google Scholar]
- Zhang XP, Li WX, Ai TS, Zou H, Wu SG, Wang GT. The efficacy of four common anthelminitic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea) Aquaculture. 2014;420:302–307. doi: 10.1016/j.aquaculture.2013.09.022. [DOI] [Google Scholar]