Abstract
To further understand the proposed signal transduction pathway involving the presumed redox proteins RdxBH and cbb3 cytochrome oxidase in Rhodobacter sphaeroides 2.4.1, a series of mutants lacking components of both the Prr two-component activation system and the cbb3-type cytochrome oxidase or RdxBH were constructed. We report that under highly aerobic conditions, aberrant photosynthesis gene expression and spectral complex formation typical of cbb3- or RdxBH-deficient mutants were no longer observed when either prrA (encoding the response regulator of the Prr system) or prrB (encoding the presumed sensor kinase) was also deleted. These double-mutant strains are phenotypically identical to single-mutant PrrA and PrrB strains, suggesting that the signal(s) originating from the cbb3 terminal oxidase affects downstream puc and puf operon expression by acting exclusively through the Prr system. When the same double-mutant strains were examined under anaerobic dark dimethyl sulfoxide growth conditions, photosynthesis gene expression was obligatorily linked to the two-component activation system. However, photosynthesis gene expression under the same growth conditions was significantly higher in the cbb3 mutant strain when compared to that in the wild type. Similarly, under anaerobic photosynthetic conditions the high levels of the oxidized carotenoid, spheroidenone, which accumulate in cbb3-deficient mutants were nearly restored to normal in a PrrB− CcoP− double mutant. This observation, together with previously published results, suggests that the regulation of the CrtA-catalyzed reaction possesses both transcriptional and posttranscriptional regulatory effectors. We propose that the cbb3 cytochrome oxidase, which by definition can interact with external oxygen, serves to control the activity of the Prr two-component activation system under both aerobic and anaerobic conditions. Although independent from the cbb3 oxidase, the RdxBH proteins are also required for normal functioning of the Prr two-component activation system and are therefore believed to lie between the cbb3 oxidase in this oxygen-sensing, redox signaling pathway and the Prr activation system.
The facultative photoheterotroph Rhodobacter sphaeroides 2.4.1 displays a remarkable ability to grow under a variety of environmental conditions, being capable of aerobic, anaerobic, and photosynthetic growth and of fixing atmospheric nitrogen and carbon dioxide (15). Significant progress has been made in understanding molecular aspects of the regulatory pathways which make this adaptability possible, and many of the key components involved in the regulation of photosystem formation have been identified. However, much less is known about interactions between the regulatory pathways themselves.
At least four such regulatory pathways in R. sphaeroides are known to be responsive to oxygen, with the FnrL protein and the Prr two-component system acting in a positive manner to control the expression of many pigment and light-harvesting-complex genes (4–6, 38), while the repressor PpsR negatively regulates photosynthesis gene (PS gene) expression (9). The TspO regulatory network appears to generate a signal through which a number of pigment biosynthesis genes and the puc operon are also negatively regulated (33, 34). Recently, we reported that the membrane-bound RdxBH proteins and the cbb3 cytochrome oxidase are involved in providing a signal through which aerobic photosynthesis gene expression is repressed. These same redox-active proteins also play a role in governing the relative accumulation of the carotenoids (Crt) spheroidene (SE) and spheroidenone (SO) under anaerobic conditions (24). Because the cbb3-generated signal normally acts to inhibit PS gene expression under aerobic conditions, it was reasoned that its target is a positive-acting regulatory effector, namely the Prr system.
The genetic region encoding the prr locus comprises three genes. The prrA gene is predicted to encode a cytoplasmic regulator, while prrB appears to encode a membrane-bound histidine kinase. Together, PrrA and PrrB represent a two-component signal transduction system. PrrA mutants are photosynthetically incompetent, while PrrB mutants do grow photosynthetically, but only under high light intensity. It was genetically shown that PrrA can be activated by phosphoryl donors other than PrrB (6, 10). The prrC gene is predicted to encode a membrane-associated protein somewhat analogous to a yeast cytochrome oxidase assembly factor (5); however, a specific role for PrrC has not yet been identified, although it does appear to be involved in this signal transduction pathway, as observed when deletions affecting different lengths of prrC are used (5). It has previously been proposed that PrrB may directly sense oxygen or the redox state of some other membrane component(s) (5); however, the precise mechanism(s) through which oxygen influences PrrB activity remains to be determined.
In order to examine the relationship between Prr- and cbb3-RdxBH-dependent PS gene expression and carotenoid biosynthesis, we have constructed a series of mutants lacking components of the Prr system and either the cbb3-type cytochrome oxidase or the RdxBH proteins. The production of both light-harvesting complexes and the transcriptional activity of the puc and puf operons in cbb3-PRR double mutants have been examined under aerobic and anaerobic conditions. By using a CCOP1PRRB1 double mutant, the accumulation of the carotenoids SE and SO under high-light photosynthetic conditions was also measured. The data presented in this paper indicate that the RdxBH proteins and cbb3 cytochrome oxidase can be categorized as new members of a complex pathway, represented by the Prr two-component system, which plays a central role in the regulation of PS gene expression and photopigment biosynthesis. Together, these constitute a sensor-signal transduction pathway, positively regulating PS gene expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and DNA manipulations.
Strains and plasmids used in this work are described in Table 1. Escherichia coli strains were grown at 37°C on LB medium (19) supplemented, when required, with the following antibiotics: tetracycline, 15 μg/ml; ampicillin, 50 μg/ml; kanamycin, 50 μg/ml; streptomycin and spectinomycin, 50 μg/ml each. R. sphaeroides 2.4.1 strains were grown at 30°C on Sistrom’s medium A (SIS) (1) containing succinate as the carbon source and supplemented as required with the following antibiotics: tetracycline, 1 μg/ml; kanamycin, 25 μg/ml; trimethoprim, 50 μg/ml; streptomycin and spectinomycin, 50 μg/ml each. Chemoheterotrophic cultures were grown aerobically on a rotary shaker or sparged with 30% O2–69% N2–1% CO2. Photosynthetic cultures were grown at high incident light intensity of 50 W/m2 and sparged with 98% N2–2% CO2. Strains grown anaerobically were cultured in SIS supplemented with 0.1% yeast extract in the presence of dimethyl sulfoxide (DMSO).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| R. sphaeroides | ||
| 2.4.1 | Wild type | W. Sistrom |
| RDXB1 | rdxB::ΩTpr | 24 |
| CCOP1 | ccoP::ΩTpr | 24 |
| PRRA2 | ΔprrA::ΩSpr Str PS− RC− B875− B800-850− Crt− | 5 |
| PRRB1 | ΔprrB::ΩSpr Str PS− | 5 |
| CCOP1PRRA2 | ccoP::ΩTpr ΔprrA::ΩSpr Str | This study |
| CCOP1PRRB1 | ccoP::ΩTpr ΔprrB::ΩSpr Str | This study |
| RDXB1PRRA2 | rdxB::ΩTpr ΔprrA::ΩSpr Str | This study |
| RDXB1PRRB1 | rdxB::ΩTpr ΔprrB::ΩSpr Str | This study |
| E. coli | ||
| DH5αphe | (φ80dlacZΔM15) ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 phe::Tn10dCm | 4 |
| S17-1 | Pro− Res− Mod+recA; integrated plasmid RP4-Tc::Mu-Km::Tn7 | 29 |
| Plasmids | ||
| pRK415 | Mob+lacZα Tcr IncP | 16 |
| pBS | Apr | Stratagene |
| pHP45Ω | Source of ΩSpr Str cassette | 26 |
| pUI1680 | Derivative of pBS; source of ΩTpr cassette | J. Eraso |
| pSUP203 | pBR325 Mob+ Apr Cmr Tcr ColE1 | 29 |
| pJE1024 | pUI1643 derivative containing internal 500-bp BstBI-PstI deletion in prrA gene; used in construction of PRRA2 strain | 5 |
| pCF200Km | Spr Str Kmr IncQ puc::lacZYA′ | 18 |
| pUI1663 | Spr Str Kmr IncQ puf::lacZYA′ | 6 |
| pUI1621 | 0.8-kb fragment from cosmid 533 containing the prrA gene in pRK415 derivative with ΩSpr Str cassette (HindIII) | 4 |
| pUI1649 | 1.9-kb SmaI-ClaI fragment containing prrB in pRK415 | 5 |
| pUI1643 | 4-kb BamHI-HindIII fragment containing prrBCA in pBS | 5 |
| pUI1653 | pUI1643 derivative containing internal 1,037-bp NruI-RsrII deletion in prrB gene; used in construction of PRRB1 strain | 5 |
DNA manipulations and analysis.
Standard protocols or manufacturer’s instructions were followed to isolate plasmid DNA, as well as for restriction endonuclease, DNA ligase, and other enzymatic treatments of plasmids and DNA fragments. Enzymes were purchased from New England Biolabs, Inc. (Beverly, Mass.), Promega Corp. (Madison, Wis.), United States Biochemical Corp. (Cleveland, Ohio), Boehringer Mannheim Biochemicals (Indianapolis, Ind.), and Bethesda Research Laboratories Life Technologies Inc. (Gaithersburg, Md.).
Conjugation techniques.
Plasmids were mobilized in biparental matings from E. coli S17-1 strains into R. sphaeroides strains as described elsewhere (2).
Construction of R. sphaeroides mutants.
The prrA and prrB genes were mutated individually in the RDXB1 and CCOP1 backgrounds. Construction of the RDXB1 and CCOP1 mutations is described elsewhere (24). Plasmids used in the construction of the PRRA2 and PRRB1 strains (5) were again employed in the disruption of the prrA and prrB genes in CCOP1 and RDXB1. CCOP1 strains contain an ΩTp insertion into the PstI site 584 bp from the start of the structural gene, and RDXB1 strains have an ΩTp cassette in the EcoRI site 240 bp from the 5′ end of the structural gene. The PRRA2 and PRRB1 strains contain internal deletions in the respective genes. Thus, PRRA2 contains a 500-bp BstBI-PstI internal deletion in the prrA gene, and PRRB1 contains a 1,037-bp RsrII-NruI internal deletion in the prrB gene. The genomic structures of the mutants were confirmed by Southern hybridizations, which were performed as previously described (3).
Southern hybridization analysis.
Hybridizations were performed according to the protocol described for Quickhyb rapid hybridization solution (Stratagene). DNA probes were oligolabeled with [α-32P]dCTP, using a random primer (New England Biolabs) and the Sequenase enzyme (United States Biochemical) as described elsewhere (28).
Spectral analysis of membrane fractions.
Crude cell-free lysates were prepared by sonication for 1 min in ICM buffer (10 mM KPO4, 1 mM EDTA; pH 7.2) with a Sonifier cell disrupter (Branson Sonic Power Co., Danbury, Conn.) followed by two rounds of centrifugation in a bench top microcentrifuge (CENTRA MP4R; International Equipment Co.) at 13,000 rpm to remove cell debris. Spectra were recorded with a UV 1601PC spectrophotometer (Shimadzu Corp.). The amount of bacteriochorophyll (Bchl) present in the B800-850 and B875 light-harvesting complexes was determined by methods that have been described elsewhere (17, 20). All analyses were performed in duplicate at least twice, and the data presented are the averages of the values obtained. Duplicate values vary by approximately ±15%.
Carotenoid and Bchl analyses.
Photopigments were extracted with acetone-methanol (7:2, vol/vol) from cell pellets and quantitated as described previously (1). The acetone-methanol-extracted pigments were then concentrated for high-performance liquid chromatography analysis on a Shimadzu system equipped with an SPD-M10AV diode array detector as described by Yeliseev et al. (32). All values are the results of duplicate assays involving duplicate experiments and vary by approximately ±10% from the mean.
β-Galactosidase assays.
R. sphaeroides cultures used for the determination of β-galactosidase activity were grown chemoheterotrophically, with sparging with 30% O2–69% N2–1% CO2, to an optical density at 660 nm (OD660) of ∼0.15. The assays were performed as described elsewhere (30). Reagent-grade o-nitrophenyl-β-d-galactopyranoside, purchased from Sigma Chemical Co., was used as the substrate. The data provided are the averages of at least two separate experiments, each performed in duplicate. Values vary by approximately ±20% from the mean.
Protein determination.
Protein concentration in crude cell extracts used for spectral analysis or β-galactosidase activity was measured by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
RESULTS
Construction of prr mutations in RDXB1 and CCOP1.
Mutations in the prrA and prrB genes and in the ccoP and rdxB genes have previously been constructed and analyzed in this laboratory (4, 5, 24). For this study individual deletions of the prrA and prrB genes were constructed in R. sphaeroides RDXB1 and CCOP1 mutant backgrounds as described in Materials and Methods. Disruption of any prr gene in the RDXB1 or CCOP1 strains was accompanied by a dramatic reduction in the deep red colony pigmentation of the parental strains, as observed on SIS agar plates in the presence of oxygen. This is similar to earlier observations on the effect of prr mutations in an otherwise wild-type strain except that the colony pigmentation of wild-type cells in the presence of oxygen is light red (4, 5), as opposed to the deep red colony pigmentation in the RDXB1 and CCOP1 mutant strains. Thus, colonies with increasingly less pigmentation were generated following deletion of the prrB and prrA genes, in that order, irrespective of whether these mutations were constructed in the wild-type, RDXB1, or CCOP1 strains. In either case, the final colony pigmentation was strictly dependent upon the status of the appropriate prr gene. This was the first indication that mutations in the prr genes have a dominant effect, relative to alteration of either the cco or rdxBH genes, on the regulation of photosystem formation under aerobic conditions. We have previously proposed that the rdxBHIS and ccoNOQP gene products encode independent members of the same redox signaling pathway (24). Our observations here, that similar phenotypes are associated with either CCOP1PRR or RDXB1PRR double mutants, are consistent with this proposal. In order to be succinct, the remainder of this report will be limited to a description of data obtained from the analysis of CCOP1PRR double mutants. However, although not presented, analysis of key RDXB1PRR double mutants was also performed and confirmed that these mutants behaved identically to the CCOP1PRR double mutants.
Oxygen regulation of photosynthetic membrane formation in CCOP1PRR mutant strains.
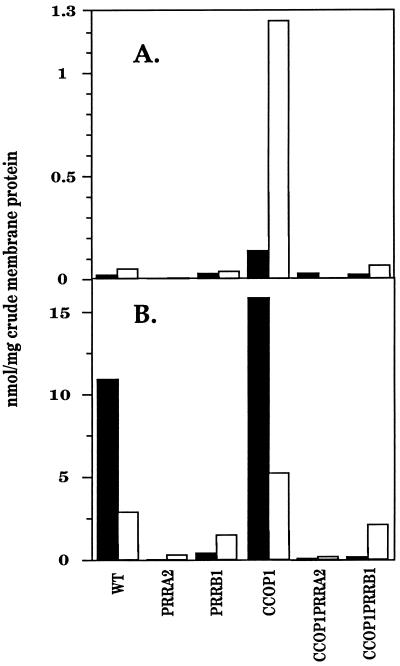
Under aerobic conditions the loss of intense coloration in colonies of CCOP1PRR double mutants compared with the deep red coloration of the CCOP1 mutant suggested that the oxygen-insensitive PS gene expression characteristic of the CCOP1 mutant was abolished in strains lacking the Prr two-component system. To investigate this further, membrane fractions from cultures of CCOP1PRRA2 and CCOP1PRRB1, grown in the presence of 30% oxygen to low cell densities to ensure fully oxygenated cultures, were prepared and spectral analysis was performed (Fig. 1A). For comparison, membrane fractions were also prepared from the PRRA2 and PRRB1 mutant strains grown under the same conditions. In the wild-type, PRRA2, and PRRB1 strains, only background levels of the B800-850 and B875 spectral complexes were produced under aerobic conditions. This contrasts with the CCOP1 mutant, which synthesizes substantial levels of both spectral complexes, especially the B875 complex, under these same conditions. Consistent with the observation that CCOP1PRR double-mutant strains have diminished pigmentation, levels of B800-850 and B875 in the double-mutant strains were also dramatically reduced when compared to those in the CCOP1 strain. This was particularly obvious for the CCOP1PRRA2 and CCOP1PRRB1 strains, where B800-850 and B875 levels were reduced to near background levels. These data provided the first direct evidence that oxygen-insensitive production of photosynthetic complexes in the CCOP1 mutant under aerobic growth is dependent upon an intact Prr two-component activation system.
FIG. 1.
Spectral complex formation under aerobic (A) and anaerobic (B) conditions of growth. Cells were grown as described in Materials and Methods and harvested at OD600 values of approximately 0.2 and 0.5, respectively. The amounts of light-harvesting complexes were expressed as nanomoles of spectral complex per milligram of crude membrane protein. Experiments were performed in duplicate, and standard deviations were ≤15% (Table 2). Filled bars represent LHII (B800-850 light-harvesting complex); open bars represent LHI (B875 light-harvesting complex).
Photosynthetic membrane formation in CCOP1PRR mutants grown anaerobically in the dark.
Having examined photosynthetic membrane formation under aerobic conditions, when the synthesis of such membranes is normally repressed, we next examined anaerobic growth conditions, when spectral complexes are normally produced by the wild type. Strains were grown anaerobically in the dark using DMSO as an external electron acceptor. These conditions were chosen because they are gratuitous for photosynthetic membrane formation and mutants lacking the prrB or prrA gene grow very poorly or not at all under photosynthetic conditions (4, 5).
The data obtained were qualitatively consistent with the aerobic data (Fig. 1B, note change in scale), although as expected the levels of spectral complexes under dark DMSO growth were substantially increased over those in cells grown in oxygen. Levels of the B800-850 and B875 spectral complexes were significantly elevated (individual determinations of spectral complex levels do not vary by more than ±15%) in the CCOP1 strain even when compared to the wild type (1.45- and 1.80-fold, respectively), despite the absence of oxygen. Disruption of the prrA or prrB gene in either the wild-type or CCOP1 backgrounds led to substantial decreases in the levels of spectral complexes. As observed for these strains when grown aerobically, the effect was most pronounced in PrrA mutants and to a lesser extent in the PrrB mutant. These data also indicate that activation of PS gene expression under anaerobic conditions by the Prr system is a prerequisite, whether in the wild type or in the CCOP1 mutant, for spectral complex formation when the PpsR repressor system is intact (11). The fact that the B800-850 spectral complex is in excess of the B875 complex under anaerobic dark DMSO conditions when compared to the relative proportion of each complex produced aerobically is due to two factors, the hierarchy of spectral complex formation when Bchl is limiting (12, 23) and the relative increased abundance of SO and its effect on B800-850 formation (32).
Analysis of PS gene expression in CCOP1PRR mutants.
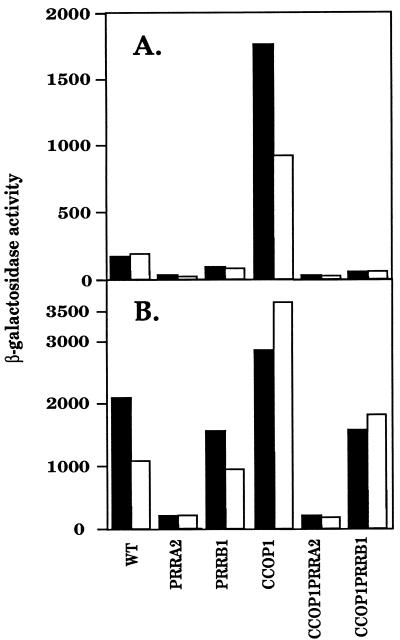
The absence or reduction in the levels of photosynthetic complexes in the CCOP1PRR mutant strains grown under both aerobic and anaerobic growth conditions when compared to those in the parental CCOP1 strain suggested that at the least, transcription of genes encoding the B800-850 and B875 structural polypeptides (encoded by the puc and puf operons, respectively) were likely to be affected. Determined with lacZ transcriptional fusions, the data for β-galactosidase levels derived from aerobically grown cells for the puc and puf operons are presented in Fig. 2A and the anaerobic β-galactosidase activities are presented in Fig. 2B. Disruption of the prrA gene in either the wild type or CCOP1 strains resulted in below-background levels of puc and puf operon activity. Mutation of prrB was similar in its effect on puc and puf operon expression as was prrA under aerobic conditions, but the mutation of prrB was far less effective under anaerobic conditions, which is in keeping with our previous characterization of these genes and their products (5). Thus, the levels of the B800-850 and B875 spectral complexes roughly correlate with puc and puf operon transcriptional activity, respectively, under either aerobic or anaerobic conditions in the presence of the cco mutation, bearing in mind that critical steps in Bchl synthesis are likely subject to other control elements (9, 37). These data provided further evidence for the role of the Prr two-component activation system in the regulation of PS gene expression in response to alterations in the cbb3 cytochrome oxidase. The relatively high levels of puc and puf operon expression under anaerobic conditions in the presence of the prrB mutation is most likely a reflection of PrrA activation by other phosphoryl donors (6, 10). Finally, it should be noted that the level of both puc and puf operon expression in the cbb3 mutant background is significantly higher (∼1.36- and 3.40-fold, respectively [±20%]) than that in the wild type even under anaerobic conditions, although the increase is less than that observed in aerobically grown cells (∼8.5- and 4.5-fold respectively [±20%]).
FIG. 2.
β-Galactosidase values under aerobic (A) and anaerobic (B) conditions of growth. Strains contain the puf::lacZ and puc::lacZ transcriptional fusions in pUI1663 and pCF200Km, respectively, in trans. Experiments were performed in duplicate, and standard deviations were ≤20%. Filled bars represent the puc::lacZ fusion; open bars represent the puf::lacZ fusion. β-Galactosidase values are expressed in micromoles per minute per milligram of protein.
Complementation of CCOP1PRR mutants.
Plasmids bearing the individual prrA and prrB genes were introduced in trans to complement the CCOP1PRRA2 and CCOP1PRRB1 mutants, respectively. Complementation, which was defined as the restoration of B800-850 and B875 to levels similar to those of either the wild type or CCOP1 mutant strain containing the same complementing plasmid, was performed under both aerobic and anaerobic growth conditions (Table 2). We reasoned that if the prr genes in trans could restore production of spectral complexes to the various mutant strains, this would further reveal the obligatory nature of the Prr two-component activation system in the cbb3 sensory transduction pathway. Introduction of these plasmids into the wild-type strain led to the production of spectral complexes, albeit at low levels, under aerobic conditions, with prrA in trans having the greatest effect followed by prrB (Table 2). Similarly, introduction of these plasmids into CCOP1 also resulted in spectral complex levels under aerobic conditions similar to those obtained with the cco mutation alone. This is consistent with our previous analysis (24) of the CCOP1 and RDXB1 mutants grown aerobically, as well as of the wild type, which indicated that it is the limitation of Bchl levels (12, 23, 32) which ultimately governs the formation of spectral complexes in these strains, even though puc and puf operon expression is at or near maximal levels. Under anaerobic conditions the introduction of the prrA gene also led to enhanced production of spectral complexes in both the wild-type and CCOP1 strains (Table 2). In each case prrA and prrB could restore spectral complex production to the double-mutant strains.
TABLE 2.
Spectral complex levels of R. sphaeroides complemented with prr genes
| Strain | Spectral complex level in strain complemented witha:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pRK415b
|
prrAc
|
prrBc
|
||||||||||
| +O2
|
−O2
|
+O2
|
−O2
|
+O2
|
−O2
|
|||||||
| LHII | LHI | LHII | LHI | LHII | LHI | LHII | LHI | LHII | LHI | LHII | LHI | |
| 2.4.1 | 0.02 | 0.05 | 10.9 | 2.9 | 0.16 | 1.46 | 15.2 | 4.4 | 0.12 | 0.44 | 3.6 | 1.6 |
| PRRA2 | <0.01 | 0.0 | 0.04 | 0.3 | 0.11 | 1.08 | 5.7 | 1.9 | — | — | — | — |
| PRRB1 | 0.03 | 0.04 | 0.4 | 1.5 | —d | — | — | — | 0.05 | 0.38 | 13.7 | 3.9 |
| CCOP1 | 0.13 | 1.25 | 15.8 | 5.2 | 0.22 | 1.32 | 20.0 | 4.9 | 0.21 | 1.52 | 12.8 | 4.0 |
| CCOP1PRRA2 | 0.02 | 0.0 | 0.06 | 0.16 | 0.17 | 1.13 | 10.2 | 2.9 | — | — | — | — |
| CCOP1PRRB1 | 0.02 | 0.06 | 0.15 | 2.1 | — | — | — | — | 0.18 | 1.28 | 6.4 | 2.6 |
Cells were grown chemoheterotrophically (+O2) and sparged with a mixture of 69% N2, 30% O2, and 1% CO2 and anaerobically in the dark with DMSO as the electron acceptor (−O2). The amounts of light-harvesting complexes were determined as described previously (17) and expressed as nanomoles of spectral complex per milligram of crude membrane protein. Experiments were performed in duplicate, and standard deviations were ≤15%. LHI and LHII are the light-harvesting complexes B875 and B800-850, respectively.
pRK415 is the vector containing the complementing prr genes.
Complementing prrA and prrB genes.
—, not determined.
Crt composition in the CCOP1PRR double mutants.
Under anoxygenic photosynthetic (medium light) and diazotrophic growth conditions, CCOP1 mutants accumulate high levels of the oxidized carotenoid SO, which in the wild type accumulates primarily under aerobic and semiaerobic growth conditions (24). Further, the effect of the ccoP mutation on SO accumulation appears to derive in part from the altered posttranscriptional regulation of carotenoid biosynthesis (24). In order to investigate the effect of prr mutations on SO accumulation in the CCOP1 background, we chose to examine photopigments extracted from the CCOP1PRRB1 mutant grown under high-light photosynthetic growth conditions. Recall that prrB mutations produce sufficient levels of spectral complexes to allow such mutants to grow photosynthetically under high light (6). Mutations in the prrA gene render strains photosynthetically incompetent irrespective of light intensity. The high-light photosynthetic cultures were sparged with a gas mixture of 98% N2–2% CO2 to ensure the complete absence of oxygen. Measurement of the relative amounts of SE and SO in the photopigments was determined as described previously (32).
The data (Table 3) revealed that the carotenoid composition of the CCOP1 mutant is almost exclusively SO relative to SE. This is consistent with the previous analysis of this strain grown diazotrophically and photosynthetically (medium light intensity) (24). The almost exclusive accumulation of SO in the CCOP1 mutant compares with the accumulation of approximately equal levels of SE and SO in the wild-type strain grown under similar conditions. As in the wild type, the levels of SE and SO in the PRRB1 single mutant were also approximately equal, suggesting that mutation in the prrB gene alone does not significantly affect the relative levels of SO accumulation. In the CCOP1PRRB1 mutant, the Crt composition was only slightly altered when compared to that in the wild-type strain. Although SO remained the predominant Crt in the double mutant, very significant levels of SE were also produced. Therefore, we must assume that PrrB plays some role in crtA gene expression. Although the total levels of Crt accumulated in the PrrB mutant strains are lower than those in either the wild type or cbb3 mutant, which are similar, the distribution of SE and SO are fully consistent with the Prr system affecting the expression of the crtA gene and functioning through the cbb3-RdxBH redox carriers.
TABLE 3.
Spectral complex and Crt composition of R. sphaeroides 2.4.1 grown under high-light photosynthetic conditions
| Straina | Amt (nmol/mg of protein)b
|
SO:SE | ||
|---|---|---|---|---|
| B800-850 | B875 | Crt | ||
| 2.4.1 | 2.97 ± 0.26 | 4.19 ± 0.78 | 81.8 ± 10.6 | 1.16 |
| CCOP1 | 2.35 ± 0.26 | 3.25 ± 0.29 | 66.0 ± 8.0 | 35.70 |
| PRRB1 | 0.19 ± 0.02 | 1.88 ± 0.24 | 43.0 ± 1.8 | 0.84 |
| CCOP1PRRB1 | 0.24 ± 0.07 | 1.91 ± 0.33 | 33.0 ± 2.8 | 2.56 |
Strains were grown at high light intensity (60 W/m2), with sparging with 98% N2–2% CO2, to an OD600 of 0.1 to 0.2.
The levels of B800-850 and B875 spectral complexes were determined as described previously (17). Values are the averages of at least two independent determinations, and standard deviations from the mean are provided.
DISCUSSION
PS gene expression in R. sphaeroides 2.4.1 is dependent upon oxygen levels and light intensity. Expression of PS genes is minimal under high-oxygen conditions (∼30% O2) but increases at low oxygen levels (∼2% O2), and it reaches full expression under anaerobic conditions under low light intensity (17, 36). Not only is the concentration of oxygen in the surrounding environment the key regulatory parameter governing the expression of PS genes in R. sphaeroides 2.4.1, it additionally dictates the form and abundance of the terminal oxidase used, depending on the particular oxygen concentration. When R. sphaeroides grows under highly oxygenated conditions, the (low-oxygen-affinity) cytochrome aa3 oxidase is the predominantly used cytochrome c oxidase (13), although under these same conditions, but to a much lesser extent, R. sphaeroides can also use the cbb3 oxidase (8). The cbb3 (high-oxygen-affinity) oxidase activity, nevertheless, is dominant when cells grow under microaerobic conditions (≤2% O2), during which its expression is maximal. The cellular expression of the ccoNOQP operon, studied using lacZ transcriptional fusions, shows a 90% reduction in cco expression under high oxygen (30%) relative to that under low oxygen (2%), and this enhanced expression is FnrL dependent (22). In this respect, the ccoNOQP operon, which encodes the cbb3 oxidase, resembles the cyd operon in E. coli, which encodes the (high-oxygen-affinity) cytochrome d oxidase and whose expression is also maximal under microaerobic conditions (31). Thus, like E. coli, R. sphaeroides varies the synthesis of its respiratory enzymes in response to oxygen availability. In addition to two cytochrome c oxidases, genes for two quinol oxidases have recently been identified in R. sphaeroides (21). They are predicted to be responsible for allowing an R. sphaeroides cytochrome bc1 complex mutant to grow under aerobic conditions (35), since bc1 is believed to be the obligatory electron donor to both the aa3 and cbb3 oxidases in R. sphaeroides under aerobiosis.
We have reported previously that mutation of the ccoNOQP operon leads to high-level PS gene expression, as well as spectral complex formation, under highly aerobic growth conditions (24), when the cells are primarily dependent upon the cytochrome aa3 terminal oxidase for aerobic growth (13). Because such cells are still subject to light-dependent regulation of PS gene expression when grown anaerobically, it was reasonable to conclude that the PpsR repressor-AppA antirepressor system was functioning normally (11). This was also borne out by the fact that the PpsR target gene, bchF, was not affected in the cco background (24). On the other hand, previous studies in our laboratory (4, 5) revealed that changes in the histidine kinase-phosphatase PrrB, or extra copies of the response regulator PrrA, which together positively regulate PS gene expression, could lead to PS gene expression and spectral complex formation under highly aerobic conditions. Thus, it was reasonable to conclude that there was likely to exist a relationship between the redox-responsive cbb3-RdxBH and the Prr activation system.
The data presented here clearly show that under aerobic growth conditions, both PS gene expression and spectral complex formation in the cbb3 mutant background are under the control of the Prr regulatory system. This two-component system is normally unable to stimulate PS gene expression in the presence of a functional cbb3-RdxBH, and therefore, Prr activation lies downstream of, but in the same pathway as, the cbb3-RdxBH redox carriers. At this point we cannot conclude that there exists a direct physical interaction between cbb3-RdxBH and the membrane-localized PrrB, although a functional interaction seems clear. Therefore, we propose that cbb3 is the primary element, or sensor of oxygen, in this regulatory pathway through its interaction with oxygen and that whatever “signal” is generated can be transmitted, we presume, to RdxBH. Alteration of RdxBH also results in aerobic expression of PS genes and spectral complex formation without affecting the activity of cbb3 (24). We presume RdxBH to lie downstream of cbb3 but upstream of Prr since the results observed here are similar regardless of whether cbb3 or RdxBH mutants are studied. The signal coming through the cbb3-RdxBH sensor-signal generator will either inhibit the kinase activity of PrrB or increase its phosphatase activity or both, resulting in the lack of activation of PrrA under aerobic conditions. The complementation results unmistakenly place the Prr system as an obligatory intermediate in the cbb3-RdxBH redox sensing-signaling pathway.
We further suggest that as oxygen becomes limiting, increased expression of ccoNOQP, requiring FnrL (22) and, we presume, rdxBH, leads to increased levels of the cognate redox carriers. Increased levels of these redox carriers, as oxygen concentrations diminish, will lead to both continued respiratory growth and a gradual diminution, we imagine, in the “strength” of the signal which negatively affects PrrB. Decreasing oxygen levels would normally result in a loss of that signal, but increased cbb3, etc., moderate that loss so that PS gene activation is gradual and not abrupt.
In the absence of cbb3, expression of the puf and puc operons under aerobic conditions is near maximal levels (compare Fig. 2A and B), while the levels of spectral complexes under aerobic conditions do not reflect the levels of puf and puc operon expression (compare Fig. 1A and B). This paradox is resolved if we consider that Bchl levels continue to remain limiting in the cbb3 mutant under aerobic as opposed to anaerobic conditions. This limitation could reflect either the activity of the PpsR repressor (9) or the low-level expression of specific genes which may require FnrL for their activation and which we believe are involved in tetrapyrrole and Bchl biosynthesis (37) or both.
These data also reveal that in the absence of an intact cbb3-RdxBH, the Prr pathway is effective, even under anaerobic conditions, in leading to increased puc and puf operon expression relative to that in the wild type (Fig. 2) and that this enhanced expression of puc and puf is through PrrA. As we have observed previously (24), and now again (also see below), cbb3-RdxBH must possess some form of activity (presumably as redox carriers) even in the absence of oxygen. That is, there must be electron flow through cbb3 under anaerobic conditions, but the ultimate acceptor remains unknown. This is in line with the observation that ccoNOQP is expressed in photosynthetic growth to a level about twofold greater than that expressed in the presence of high oxygen (22). What is equally striking about these results is that the PpsR repressor-AppA antirepressor system (11) does not appear to play a major role in puc and puf operon expression when the signal that presumably keeps PrrB “inactive” is removed.
These data further support our earlier findings that PrrA can be activated through the intervention of heterologous signal transduction pathways (6), particularly under anaerobic conditions (compare Fig. 2A and B). This observation most likely relates to the role of PrrA in those activities which are derivative of the photosynthetic lifestyle, e.g., the uptake hydrogenase (10), CO2 fixation, N2 fixation (15), and presumably others. By inference, these results suggest that the cbb3-RdxBH oxygen sensor-signal generator also plays a role in the expression of these “dependent” metabolic pathways. However, these pathways, e.g., N2 fixation, may possess additional regulatory elements, as does PS gene expression, such as PpsR or FnrL.
We should return to the question of how the cbb3-RdxBH sensor-signal generator pair communicates with PrrB, which we presume to be the responsive element in this activation pathway. One possibility is through some membrane-localized redox flux. In this sense, PrrB resembles more the sensor histidine kinase-phosphatase ArcB in E. coli, which has been proposed to sense oxygen by the level of either an electron transport component in reduced form or another compound linked to the process by a redox reaction (14). Thus, photosynthesis and respiration might emerge as being coordinately regulated, and might share common components, as has been previously suggested (27). Another possibility is through the PrrC protein; the presence of extra copies of the prrC gene actually results in modest inhibition of PS gene expression (5). A third possibility is through some element(s) of the cbb3 terminal oxidase, e.g., the Q gene product, whose absence has no apparent effect on cbb3 oxidase activity (25), or through some other, as yet unidentified activity or component. Interestingly, the Rhodobacter capsulatus equivalent of PrrC, namely SenC, when inactivated, has an effect upon cbb3 activity, which is not true in R. sphaeroides (7).
In this report (Table 3) and elsewhere (33), the data suggest that crtA gene expression is regulated at the transcriptional level through the cbb3-RdxBH–Prr pathway. However, earlier results (24) also revealed that crtA activity was also likely to be under posttranscriptional control. It was shown that in the cbb3 mutant strain the level of SE decreased by a factor of 3, but a crtA::lacZ fusion showed only an ∼55% increase in lacZ expression in the cbb3 mutant relative to that in the wild type.
The tools appear to be at hand to address all of these questions, and it is apparent that PS gene regulation is inextricably linked to numerous dependent pathways which together constitute the photosynthesis lifestyle.
ACKNOWLEDGMENTS
This work was supported by USPHS grant GM15590 to S.K.
We thank Nigel Mouncey for helpful discussions.
REFERENCES
- 1.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1957;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 2.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a Puf− mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donohue T J, McEwan A G, Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986;168:962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eraso J M, Kaplan S. Complex regulatory activities associated with the histidine kinase PrrB in expression of photosynthesis genes in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1996;178:7037–7046. doi: 10.1128/jb.178.24.7037-7046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eraso, J. M., and S. Kaplan. 1997. Unpublished results.
- 8.Garcia-Horsman J A, Berry E, Shapleigh J P, Alben J O, Gennis R B. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 9.Gomelsky M, Kaplan S. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchF expression. J Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomelsky M, Kaplan S. Isolation of regulatory mutants in photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1 and partial complementation of a PrrB mutant by the HupT histidine-kinase. Microbiology. 1995;141:1805–1819. doi: 10.1099/13500872-141-8-1805. [DOI] [PubMed] [Google Scholar]
- 11.Gomelsky M, Kaplan S. A molecular genetic analysis suggesting interactions between AppA and PpsR in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong L, Lee J, Kaplan S. The Q gene of Rhodobacter sphaeroides: its role in puf operon expression and spectral complex assembly. J Bacteriol. 1993;176:2946–2961. doi: 10.1128/jb.176.10.2946-2961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosler J P, Fetter J, Tecklenberg M M J, Espe M, Lerma C, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase: purification, kinetics, proton pumping and spectral analysis. J Biol Chem. 1992;267:24264–24272. [PubMed] [Google Scholar]
- 14.Iuchi S, Chepuri V, Fu H-A, Gennis R B, Lin E C C. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990;172:6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 17.Kiley P J, Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988;52:50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J K, Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 20.Meinhardt S W, Kiley P J, Kaplan S, Crofts A R, Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985;236:130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- 21.Mouncey, N. J., M. Choudhary, and S. Kaplan. 1997. Unpublished observations.
- 22.Mouncey N J, Kaplan S. Oxygen regulation of the ccoN gene encoding cbb3 oxidase in Rhodobacter sphaeroides 2.4.1T: involvement of the FnrL protein. J Bacteriol. 1998;180:2228–2231. doi: 10.1128/jb.180.8.2228-2231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neidle E L, Kaplan S. 5-Aminolevulinic acid availability and control of spectral complex formation in HemA and HemT mutants of Rhodobacter sphaeroides. J Bacteriol. 1993;175:2304–2313. doi: 10.1128/jb.175.8.2304-2313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Gara J P, Kaplan S. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:1951–1961. doi: 10.1128/jb.179.6.1951-1961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preisig O, Zufferey R, Thony-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 27.Scherer S. Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem Sci. 1990;15:458–462. doi: 10.1016/0968-0004(90)90296-n. [DOI] [PubMed] [Google Scholar]
- 28.Sen P, Murai N. Oligolabeling DNA probes to high specific activity with sequenase. Plant Mol Biol Rep. 1991;9:128–131. [Google Scholar]
- 29.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 30.Tai T-N, Havelka W A, Kaplan S. A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid. 1988;19:175–188. doi: 10.1016/0147-619x(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 31.Tseng C-P, Albrecht J, Gunsalus R P. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeliseev A A, Eraso J M, Kaplan S. Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides 2.4.1: involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J Bacteriol. 1996;178:5877–5883. doi: 10.1128/jb.178.20.5877-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeliseev A A, Kaplan S. A sensory transducer homologous to mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 34.Yeliseev A A, Krueger K E, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc Natl Acad Sci USA. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun C-H, Beci R, Crofts A R, Kaplan S, Gennis R B. Cloning and DNA sequencing of the fbc operon encoding the cytochrome bc1 complex from Rhodobacter sphaeroides. Eur J Biochem. 1990;194:399–411. doi: 10.1111/j.1432-1033.1990.tb15633.x. [DOI] [PubMed] [Google Scholar]
- 36.Zeilstra-Ryalls J, Gomelsky M, Eraso J M, Yeliseev A, O’Gara J, Kaplan S. Control of photosystem formation in Rhodobacter sphaeroides. J Bacteriol. 1998;180:2801–2809. doi: 10.1128/jb.180.11.2801-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeilstra-Ryalls J H, Gabbert K, Mouncey N J, Kaplan S, Kranz R G. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J Bacteriol. 1997;179:7264–7273. doi: 10.1128/jb.179.23.7264-7273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]