Abstract
Background
Spirulina [SPIR] (cyanobacterium) and chlorella [CHLO] (microalgae) are foods rich in protein and essential amino acids; however, their capacity to stimulate myofibrillar protein synthesis (MyoPS) in humans remains unknown.
Objectives
We assessed the impact of ingesting SPIR and CHLO compared with an established high-quality nonanimal-derived dietary protein source (fungal-derived mycoprotein [MYCO]) on plasma amino acid concentrations, as well as resting and postexercise MyoPS rates in young adults.
Methods
Thirty-six healthy young adults (age: 22 ± 3 y; BMI: 23 ± 3 kg·m-2; male [m]/female [f], 18/18) participated in a randomized, double-blind, parallel-group trial. Participants received a primed, continuous infusion of L-[ring-2H5]-phenylalanine and completed a bout of unilateral-resistance leg exercise before ingesting a drink containing 25 g protein from MYCO (n = 12; m/f, 6/6), SPIR (n = 12; m/f, 6/6), or CHLO (n = 12; m/f, 6/6). Blood and bilateral muscle samples were collected at baseline and during a 4-h postprandial and postexercise period to assess the plasma amino acid concentrations and MyoPS rates in rested and exercised tissue.
Results
Protein ingestion increased the plasma total and essential amino acid concentrations (time effects; all P < 0.001), but most rapidly and with higher peak responses following the ingestion of SPIR compared with MYCO and CHLO (P < 0.05), and MYCO compared with CHLO (P < 0.05). Protein ingestion increased MyoPS rates (time effect; P < 0.001) in both rested (MYCO, from 0.041 ± 0.032 to 0.060 ± 0.015%·h−1; SPIR, from 0.042 ± 0.030 to 0.066 ± 0.022%·h−1; and CHLO, from 0.037 ± 0.007 to 0.055 ± 0.019%·h−1, respectively) and exercised tissue (MYCO, from 0.046 ± 0.014 to 0.092 ± 0.024%·h−1; SPIR, from 0.038 ± 0.011 to 0.086 ± 0.028%·h−1; and CHLO, from 0.048 ± 0.019 to 0.090 ± 0.024%·h−1, respectively), with no differences between groups (interaction effect; P > 0.05), but with higher rates in exercised compared with rested muscle (time × exercise effect; P < 0.001).
Conclusions
The ingestion of a single bolus of algae-derived SPIR and CHLO increases resting and postexercise MyoPS rates to a comparable extent as MYCO, despite divergent postprandial plasma amino acid responses.
Keywords: algae, mycoprotein, amino acids, muscle protein synthesis, resistance exercise, stable isotopes
Introduction
Dietary protein [1] and muscle contraction [2] independently and collectively [3] stimulate muscle protein synthesis (MPS) rates, making them essential for supporting skeletal muscle maintenance, remodeling, and/or accretion over time. The postexercise MPS response following dietary protein ingestion is increased because of the rise and total availability of circulating essential amino acids (EAAs) [4,5], particularly leucine, which acts as both signal and substrate [6]. Different protein sources differ in terms of their EAA and leucine contents [7,8], as well as their protein digestion and amino acid absorption kinetics [[9], [10], [11]], the latter of which is strongly modulated by the particular food matrix in which the protein is embedded [12,13]. As such, the magnitude of the (postexercise) postprandial MPS response can differ following the ingestion of different protein-rich whole foods.
It has been repeatedly demonstrated that animal-derived protein sources robustly stimulate rested and postexercise MPS [9,[13], [14], [15], [16]]. However, animal-based protein production is associated with increasing ethical and environmental concerns [17], and there is growing interest in nonanimal-derived and sustainably produced protein sources [18]. Alternative dietary protein-rich sources, such as plants, fungi, and algae, typically require less water and land resources [[19], [20], [21]] and are associated with lower greenhouse gas emissions per kilogram of protein produced compared with animal-derived foods [18,22]. However, concerns have been raised as to whether nonanimal-derived protein sources are of inferior quality, on account of less favorable EAA profiles and/or digestion and absorption kinetics [7,14,15]. Therefore, larger doses may be required for an equivalent postprandial MPS response, partially mitigating any environmental benefit. However, despite a wide array of potential dietary protein sources available, empirical comparisons are so far limited to the resting and/or postexercise MPS response to only soy- [14,23], wheat- [15,24], potato- [25], or plant-based blends [24,26,27], compared with animal counterparts.
We recently identified a fungal-derived protein (mycoprotein [MYCO]) as a high-quality nonanimal-derived protein-rich whole food source, capable of robustly stimulating resting and postexercise MPS rates [[28], [29], [30]] and, as a result, supporting longer-term muscle adaptive responses [30]. An intriguing potential alternative protein source is algae. Algae are unicellular photosynthetic microorganisms that are usually cultivated under controlled environmental circumstances (e.g., temperature, pH, light, carbon dioxide, and nutrient supplies), which permits favorable environmental credentials [31]. Spirulina [SPIR] (Arthrospira platensis), a cyanobacterium or blue-green alga, and chlorella [CHLO] (Chlorella vulgaris), a microalga, are the 2 most commercially available microalgae in human nutrition, and although being produced as natural protein- (>50% of total mass) and EAA- (>40% of total protein) rich food supplements, they are typically consumed in only small amounts as a means of delivering high doses of micronutrients [18]. Consideration for the consumption of algae in sufficient quantities to fulfill human dietary protein requirements has received less attention. We recently observed that plasma EAA availability following the ingestion of SPIR was comparable to MYCO, butconsiderably more rapid and greater compared with CHLO, in both young and older adults [32]. This was likely attributable to the more robust cell structure of CHLO, making the protein less bio-accessible for human digestion and/or absorption. To our knowledge, no human investigation has reported whether such differences in postprandial amino acid kinetics following algal ingestion translate to varying anabolic potential across sources.
In the present study, we assessed the myofibrillar protein synthesis (MyoPS) responses following the ingestion of isonitrogenous (containing 25 g protein) boluses of SPIR, CHLO, or MYCO in resting and exercised muscle of healthy young adults. We hypothesized that the ingestion of SPIR would stimulate MyoPS rates to a similar extent as MYCO and more so when compared with CHLO.
Methods
Participants
Thirty-six healthy, recreationally active young adults (age, 22 ± 3 y; body mass, 70 ± 12 kg; BMI, 23 ± 3 kg·m-2; male/female: 18/18) volunteered to participate in this double-blind, randomized, controlled parallel groups design trial (Supplementary Figure 1). Participants’ characteristics are presented in Table 1. Inclusion criteria for potential participants were age 18–40 y, BMI 18.5–27.5 kg·m-2, blood pressure <140/90 mmHg, not experienced with structured resistance exercise training (≤3 times per week for ≥3 mo before participation), and not having undergone any previous stable-isotope tracer protocols in the previous 12 mo, thus ensuring negligible background stable-isotope enrichments. Exclusion criteria included any metabolic impairments, cardiovascular complications, or allergies to MYCO, edible fungi, penicillin, or algae-based foods, and smoking. All participants were informed about the experimental procedures, potential risks, and the purpose of the study before obtaining written informed consent. This study was registered as a clinical trial at clinicaltrials.gov (NCT05016557) and was approved by the Sport and Health Sciences Ethics Committee of the University of Exeter (Ref No. 200506/B/01) per standards for human research outlined in the latest version of the Declaration of Helsinki.
TABLE 1.
Participants’ characteristics1
| MYCO (n = 12) | SPIR (n = 12) | CHLO (n = 12) | P value | |
|---|---|---|---|---|
| Sex, m/f | 6/6 | 6/6 | 6/6 | n/a |
| Age, y | 22 ± 4 | 22 ± 3 | 21 ± 2 | 0.86 |
| Body mass, kg | 71 ± 11 | 70 ± 12 | 69 ± 15 | 0.92 |
| Height, cm | 174 ± 7 | 173 ± 9 | 174 ± 14 | 0.96 |
| BMI, kg/m2 | 23 ± 3 | 22 ± 2 | 23 ± 4 | 0.89 |
| Lean mass, kg | 56 ± 10 | 57 ± 14 | 56 ± 10 | 0.99 |
| Body fat, % | 21 ± 8 | 19 ± 9 | 19 ± 8 | 0.83 |
| Systolic BP, mmHg | 117 ± 7 | 122 ± 10 | 117 ± 12 | 0.37 |
| Diastolic BP, mmHg | 68 ± 7 | 71 ± 8 | 70 ± 10 | 0.71 |
| Leg press 1RM, kg | 111 ± 37 | 108 ± 41 | 115 ± 31 | 0.87 |
| Leg extension 1RM, kg | 51 ± 18 | 52 ± 19 | 54 ± 15 | 0.94 |
| Leg press volume, kg × rep | 3504 ± 1205 | 3991 ± 1981 | 3741 ± 1493 | 0.76 |
| Leg extension volume, kg × rep | 1129 ± 348 | 1401 ± 586 | 1224 ± 458 | 0.37 |
| Total exercise volume, kg × rep | 4634 ± 1458 | 5392 ± 2447 | 4965 ± 1774 | 0.63 |
Values represent mean ± SD. Data were analyzed using one-way ANOVA. Abbreviations: BP, blood pressure; CHLO, chlorella; MYCO, mycoprotein; SPIR, spirulina; 1RM, 1 repetition maximum.
Pretesting
Before inclusion in the study, participants were initially screened to assess body mass, height, blood pressure, and body composition (via Air Displacement Plethysmography [BodPod, Life Measurement, Inc.]). Participants were deemed healthy based on their responses to a routine medical screening questionnaire and screening results. Following screening and acceptance into the study, the participants were familiarized with the unilateral-resistance–type exercise protocol and exercise equipment, which was done ≥5 d before the experimental trial, as described below. Participants were instructed to report their habitual dietary intake by recording a weighed food diary for 2 weekdays and 1 weekend before participating in the study. Diaries were subsequently analyzed for energy and macronutrient intakes using dedicated software (Nutritics Ltd.).
Diet and activity before testing
All participants were instructed to refrain from any vigorous physical activity and/or exercise and maintain their usual dietary habits for 3 d before the trial, as well as refrain from alcohol consumption for 1 d before the trial. To control for the timing of food intake and variation in the participants’ habitual diet, all participants were provided with a standardized meal containing 2174 kJ of energy, with 52% of the energy provided as carbohydrate, 17% as fat, and 31% as protein, and were asked to consume it as their last food intake before 20:00 on the evening before the experimental trial. All female participants not taking hormonal contraceptives were tested during the first 10 d (early- to mid-part of the follicular phase) of their menstrual cycle to control for hormonal fluctuations, which have been previously suggested to influence protein metabolism [33].
Experimental protocol
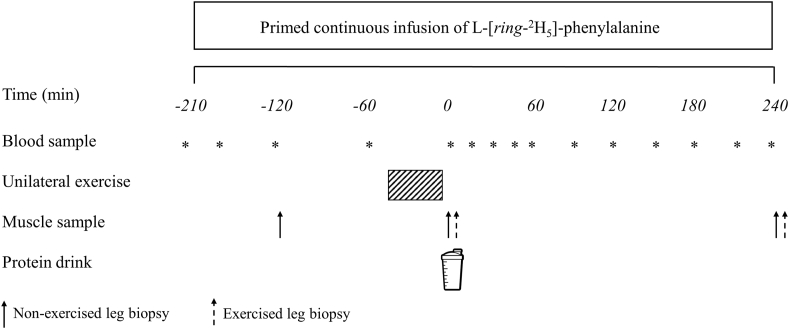
Participants were randomly assigned to 1 of 3 parallel groups by an independent researcher and completed a single experimental trial in a double-blind manner. An overview of the experimental protocol is presented in Figure 1. On the day of the experimental trial, participants arrived at the laboratory at 08:00 after an overnight fast. A polytetrafluoroethylene cannula was inserted into an antecubital vein for stable-isotope amino acid infusion. After taking a baseline venous blood sample to measure the background isotope enrichments, the phenylalanine pool was primed with a single intravenous dose of L-[ring-2H5]-phenylalanine (2.12 μmol·kg-1). Thereafter, a continuous L-[ring-2H5]-phenylalanine intravenous tracer infusion was initiated (t = −210 min) and maintained at a rate of 0.05 μmol·kg-1·min-1 for the entire test day. After the infusion was started, a second polytetrafluoroethylene cannula was inserted into a dorsal hand vein and placed within a heated hand unit (55°C) for collecting repeated arterialized venous blood samples [34]. Arterialized venous blood samples were collected throughout the experimental protocol at the following intervals: −165, −120, −60, 0, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min.
FIGURE 1.
Schematic representation of the experimental protocol.
A baseline muscle biopsy sample was collected at t = −120 min from the nondominant leg (designated as the “resting leg”). All muscle biopsies were collected from the mid-region of the M. vastus lateralis with a modified Bergstrom suction needle under local anesthesia using 2% lidocaine. All biopsy samples were immediately freed from any visible blood, adipose, and connective tissue, frozen in liquid nitrogen, and stored at −80°C until subsequent analysis. At t = −45 min, the participants were taken by wheelchair to execute a bout of unilateral leg resistance exercise, as described below. On completion of the exercise protocol, participants were taken back to the laboratory by wheelchair such that no weight bearing was performed with the resting leg throughout the experiment. Immediately after exercise, bilateral muscle biopsy samples were collected from the rested (>1 to 2 cm distal from the baseline incision) and exercised leg. Immediately following the biopsies, participants ingested a beverage corresponding to their randomly assigned treatment allocation, either a MYCO, SPIR, or CHLO drink, all of which contained 25 g protein, within 5 min. Consumption of the drink (t = 0 min) marked the commencement of the postprandial period and participants rested in a semisupine position for 4 h. At t = 5 min, participants completed a 100-mm visual analog scale to subjectively assess questions on drink palatability (taste, aftertaste, texture, and overall palatability of the drink), with 0 being “worst ever” and 100 being “best ever,” as previously described [35]. They were also asked to identify the protein beverage consumed. No differences were observed for taste, texture, and overall palatability (all P > 0.05), but the score for aftertaste was lower for CHLO than for MYCO (P = 0.03) (Supplementary Table 1). The protein beverage was identified correctly by 33% (3 of 12 in MYCO, 5 of 12 in SPIR, and 4 of 12 in CHLO), indicating partially successful blinding procedures. At t = 240 min, additional bilateral biopsies were collected 1–2 cm distal to the previous incisions, the completion of which marked the termination of the experimental test day.
Unilateral-resistance exercise protocol
During the pretesting visit, the unilateral 3 repetition max (3RM) was assessed to estimate 1RM for leg press and leg extension [36]. 3RM, rather than 1RM, was selected as an accurate approach to predict 10RM to minimize safety risks [36]. The dominant leg was used for the exercise to maximize the weight lifted, optimize movement execution, and thereby create a maximal exercise stimulus [14]. Strength testing began with a brief warm-up and practice of each exercise. Thereafter, participants attempted 3RM with a self-selected weight. The weight was increased for each subsequent attempt, with the final 3RM being accepted as the last weight lifted correctly before a failed attempt (<3 repetitions lifted correctly). Then, 10RM was calculated as 70% of the estimated 1RM. Upon the completion of 3RM testing, participants rested for ∼5 min and were then asked to complete 1 set (∼10 repetitions) at the calculated 70% 1RM for familiarization and verification.
For the experimental trial, participants completed a brief warm-up of the leg press exercise, consisting of 10 repetitions at 50% 10RM, followed by 10 repetitions at 70% 10RM performed unilaterally with the dominant leg per pretesting. Thereafter, participants executed 4 unilateral sets of leg press, followed by 4 sets of leg extension at the predetermined 10RM until volitional failure (8–12 repetitions). The weight was increased when participants could perform >12 repetitions and decreased when participants could not perform 8 repetitions, such that each set was performed due to fatigue or failure. Verbal encouragement was provided throughout, and the rested nondominant leg was kept relaxed and unloaded throughout.
Experimental beverage preparations
Freeze-dried MYCO was produced and obtained from Marlow Foods Ltd., Quorn Foods Stokesley. SPIR and cracked-cell CHLO powder were obtained from commercial suppliers (Bulk and Naturya Ltd., respectively). All protein sources were independently analyzed by a third-party company (Premier Analytical Services) for energy, macronutrient content, and amino acid composition. The powdered protein sources were prepared the evening before the experimental trial. The dried powders were dissolved in 425 mL water, 50 mL energy-free artificial vanilla flavoring (Skinny Mixes), and 25 mL energy-free green food coloring (Tesco Stores Ltd.) for blinding purposes, blended for ∼2 min and refrigerated overnight. Drinks were enriched (2%) with L-[ring-2H5]-phenylalanine to maintain systemic isotopic steady state following protein ingestion [37]. During the experimental trial, once participants had consumed the drink, an additional 100 mL water was added to rinse the bottle and ensure that all protein had been consumed, taking the total fluid volume consumption to 600 mL. All drinks were well-tolerated and consumed within the allotted 5 min (average time to consumption was 3.9 ± 2.1, 4.7 ± 1.4, and 4.0 ± 2.1 min for MYCO, SPIR, and CHLO, respectively; P = 0.52), with no adverse effects reported during or after the test day. Double-blinding of the drinks was achieved by having an independent person prepare the drinks in an opaque bottle. The drinks were matched for protein content (25 g), which required 46.7, 40.4, and 42.0 g of MYCO, SPIR, and CHLO powders, respectively. The detailed nutritional content and amino acid composition of the drinks are displayed in Table 2.
TABLE 2.
Nutritional content of the experimental drinks
| MYCO | SPIR | CHLO | |
|---|---|---|---|
| Macronutrients and energy | |||
| Protein1, g | 25 | 25 | 25 |
| Carbohydrate, g | 2.8 | 4.9 | 6.8 |
| Fat, g | 3.5 | 1.1 | 2.9 |
| Fiber, g | 11.3 | 3.6 | 3.3 |
| Energy, kJ | 692 | 576 | 675 |
| Energy, kcal | 165 | 137 | 160 |
| Amino acid content, g | |||
| Alanine | 1.4 | 2.0 | 2.1 |
| Arginine | 1.5 | 1.7 | 1.5 |
| Aspartic acid | 2.1 | 2.6 | 2.2 |
| Glutamic acid | 2.7 | 3.5 | 2.8 |
| Glycine | 1.0 | 1.3 | 1.4 |
| Histidine | 0.5 | 0.4 | 0.5 |
| Isoleucine | 1.0 | 1.4 | 0.9 |
| Leucine | 1.7 | 2.3 | 2.1 |
| Lysine | 1.7 | 1.3 | 2.0 |
| Phenylalanine | 1.0 | 1.2 | 1.2 |
| Proline | 1.0 | 0.9 | 1.2 |
| Serine | 1.1 | 1.3 | 1.0 |
| Threonine | 1.2 | 1.3 | 1.1 |
| Tyrosine | 0.8 | 1.1 | 0.9 |
| Valine | 1.2 | 1.6 | 1.4 |
| EAA | 8.2 | 9.5 | 9.2 |
| NEAA | 11.6 | 14.4 | 13.1 |
| ΣAA | 19.8 | 23.9 | 22.3 |
Protein content was calculated as nitrogen × 6.25.
Abbreviations: CHLO, chlorella; EAA, essential amino acids; MYCO, mycoprotein; NEAA, nonessential amino acids; SPIR, spirulina; TAA, total amino acids.
Analyses of blood samples and skeletal muscle tissue
Ten milliliters of arterialized venous blood were collected in a syringe at each sampling point. From each blood sample, 20 μL was collected into a plastic capillary tube and immediately analyzed for blood glucose concentrations (Biosen C-Line GP+). A second part (5 mL) was collected in lithium heparin-containing tubes (BD Vacutainer LH; BD Diagnostics) and centrifuged immediately at 4000 × g and 4°C for 10 min to obtain the plasma samples. The plasma supernatant was then removed, aliquoted, and frozen at −80°C for subsequent analysis. The remaining blood was added to fresh evacuated tube (BD Vacutainer SST II tubes, BD Diagnostics), left to clot at room temperature for >30 min, and then centrifuged at 4000 × g at 4°C for 10 min to obtain serum. The serum supernatant was then removed, aliquoted, and frozen at −80°C for subsequent analysis. Serum insulin concentrations were analyzed using a commercially available kit (DRG Insulin ELISA, EIA-2935, DRG International Inc.).
Plasma L-[ring-2H5]-phenylalanine enrichments and concentrations of phenylalanine, leucine, valine, isoleucine, lysine, histidine, glutamic acid, methionine, proline, serine, threonine, tyrosine, glycine, and alanine were determined by GC-MS with electron impact ionization (Agilent) as previously described [[37], [38], [39]]. Muscle biopsy tissue samples were analyzed for myofibrillar protein-bound L-[ring-2H5]-phenylalanine. First, the myofibrillar protein fraction was extracted, as previously described [37,39]. Next, myofibrillar protein-bound L-[ring-2H5]-phenylalanine enrichments were determined using GC-MS, as previously described [37,40].
Calculations
The fractional synthetic rate (FSR) of myofibrillar muscle protein was calculated using the standard precursor-product Equation 1 [38], as follows:
| Equation 1 |
Where ΔEp is the increment in protein-bound L-[ring-2H5]-phenylalanine in myofibrillar muscle protein between 2 biopsies, Eprecursor is the weighted mean L-[ring-2H5]-phenylalanine enrichment in the plasma over time, and t is the tracer incorporation time (h) between muscle biopsies. Total postprandial glucose, insulin, and amino acid availability were calculated as incremental AUC (iAUC) using the trapezoid rule, with the baseline set as t = 0.
Statistical analyses
Based on previous research [28], a sample size of 12 subjects per intervention, including a 10% dropout rate, was calculated using a 2-sided repeated-measures ANOVA (P < 0.05, 80% power, effect size 0.59; G∗power version 3.1.9.2) to compare FSR between groups as the primary outcome measure. Secondary outcomes included serum insulin and plasma amino acid responses, whereas the remaining outcomes were classified as tertiary. Participant characteristics, exercise volume performed, and background L-[ring-2H5]-phenylalanine enrichments were analyzed by one-way ANOVA. Differences in blood glucose concentrations, serum insulin concentrations, plasma amino acid concentrations, and plasma L-[ring-2H5]-phenylalanine enrichments were analyzed using two-way (time × group [MYCO compared with SPIR compared with CHLO]) repeated-measures ANOVA. Myofibrillar FSR and myofibrillar L-[ring-2H5]-phenylalanine enrichments were analyzed using a three-way (time × group × exercise) ANOVA. When significant interactions were observed, Bonferroni post hoc tests were performed to identify individual differences. Peak concentrations, time-to-peak, and iAUC values were analyzed using one-way ANOVA. Data were tested for sphericity, and where violations occurred, the Greenhouse-Geisser correction was automatically applied. Statistical significance was set at P value of <0.05. Statistical analyses were performed using GraphPad Prism version 9.5.0 and IBM SPSS Statistics version 28.0.0.0. All data are expressed as mean ± SD.
Results
Participants’ characteristics
Participant and exercise characteristics are presented in Table 1. No differences in any parameter were observed between groups (all P > 0.05).
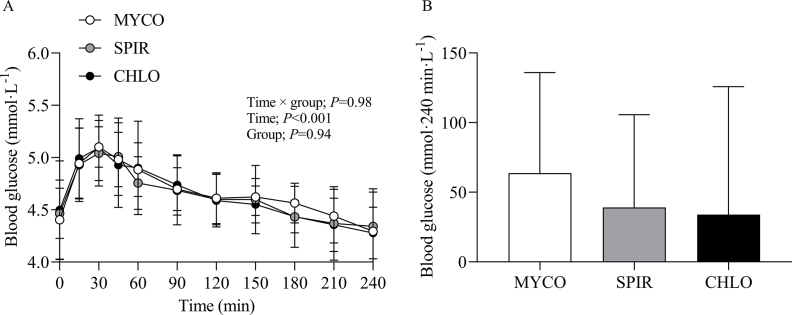
Blood glucose and serum insulin concentrations
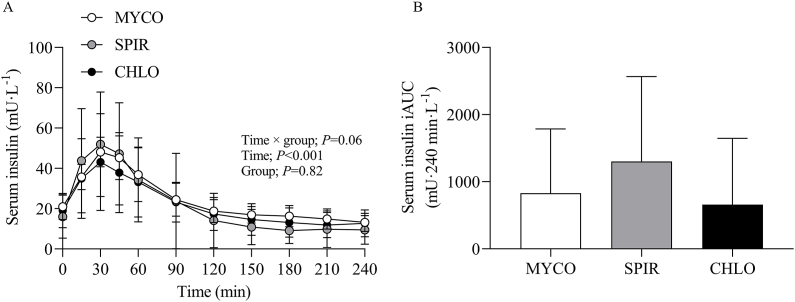
Blood glucose concentrations increased after drink ingestion and returned to postabsorptive levels by t = 120 min (time effect; P < 0.001), with no differences between groups (group and time × group interaction; P = 0.94 and 0.98, respectively). Postprandial blood glucose iAUC did not differ between groups (P = 0.61) (Supplementary Figure 2). From similar postabsorptive concentrations, serum insulin concentrations increased following drink ingestion (time effect; P < 0.001), and although no group differences were present (P = 0.82), a varyingly increasing trend was detected between groups (time × group interaction; P = 0.06). Postprandial serum insulin iAUC did not differ between groups (P = 0.33) (Figure 2).
FIGURE 2.
Serum insulin concentration over time (A) and total insulin response (B), expressed as iAUC, during the 4-h postprandial period following the ingestion of 25 g protein from mycoprotein (MYCO; n = 12), spirulina (SPIR; n = 12), or chlorella (CHLO; n = 12) and execution of a bout of unilateral-resistance leg exercise in healthy young adults. Time 0 represents the time of protein ingestion. Values are mean ± SD. Serum insulin concentration over time was analyzed with two-way repeated-measures ANOVA (time × group), and iAUC was analyzed with one-way ANOVA. Bonferroni post hoc tests were applied to detect individual differences. Time × group interaction; P = 0.06. CHLO, chlorella; iAUC, incremental area under the curve; MYCO, mycoprotein; SPIR, spirulina.
Plasma amino acid concentrations
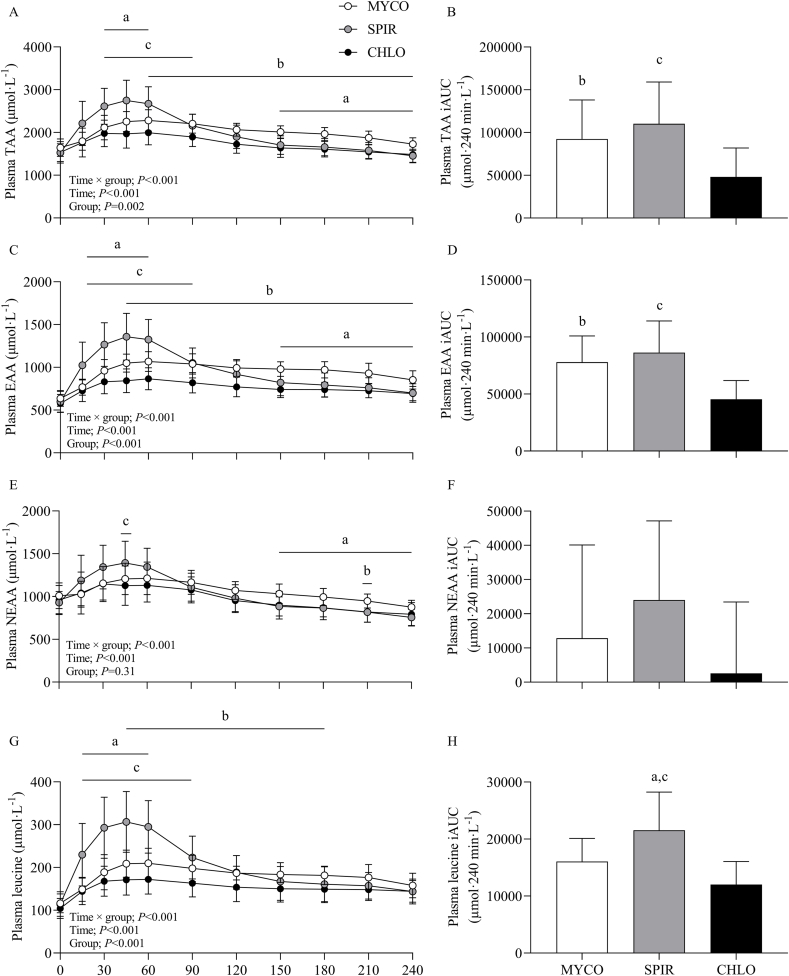
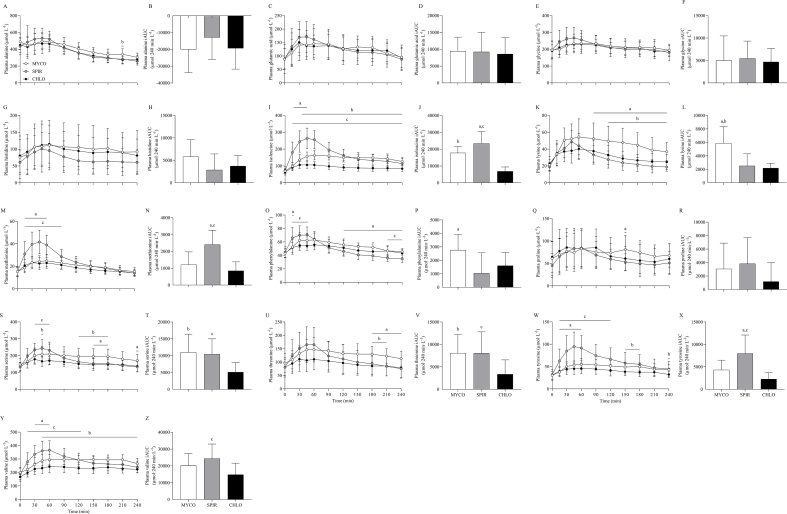
Plasma total amino acid (TAA), EAA, nonessential amino acid (NEAA), and leucine concentrations are presented in Figure 3. From similar postabsorptive concentrations, plasma TAA, EAA, and NEAA concentrations increased following drink ingestion (time effects; all P < 0.001), although to different extents between groups (time × group interactions; all P < 0.001). Postprandial plasma peak TAA concentrations rose to a greater degree in SPIR (2822 ± 438 μmol·L-1) compared with MYCO (2372 ± 244 μmol·L-1) and CHLO (2044 ± 290 μmol·L-1) (P < 0.01), and remained at higher concentrations in SPIR than in MYCO and CHLO at 30–60 min and 30–90 min, respectively (P < 0.05). In MYCO, postprandial plasma TAA concentrations were higher than those in CHLO for most of the postprandial period (60–240 min; P < 0.05), and higher than those in SPIR toward the end of the postprandial period (150–240 min; P < 0.01). Drink ingestion resulted in higher peak EAA concentrations in SPIR (1388 ± 249 μmol·L-1) compared with MYCO (1127 ± 99 μmol·L-1) and CHLO (898 ± 114 μmol·L-1) (P < 0.01), with MYCO being higher than CHLO (P < 0.01). Postprandial plasma EAA concentrations remained elevated throughout the postprandial period in all groups (except for 240 min in the SPIR group) (P < 0.01) and were higher in SPIR than in MYCO and CHLO at 15–60 min and 15–90 min, respectively (P < 0.05). For both TAA and EAA concentrations, amino acid availability over the 4-h postprandial period, as expressed by iAUC, was higher for MYCO and SPIR than for CHLO (both P < 0.05).
FIGURE 3.
Plasma TAA (A), EAA (C), NEAA (E), and leucine (G) concentrations and total TAA (B), EAA (D), NEAA (F), and leucine (H) responses over time, expressed as iAUC, during the 4-h postprandial period following the ingestion of 25 g protein from mycoprotein (MYCO; n = 12), spirulina (SPIR; n = 12) or chlorella (CHLO; n = 12) and execution of a bout of unilateral-resistance leg exercise in healthy young adults. Time 0 represents the time of protein ingestion. Values are mean ± SD. Plasma concentrations over time were analyzed with two-way repeated-measures ANOVA (time × group), and iAUCs were analyzed with one-way ANOVA. Bonferroni post hoc tests were applied to detect individual differences. Time × group interactions; all P < 0.001. a, significant difference for MYCO compared with SPIR (P < 0.05); b, significant difference for MYCO compared with CHLO (P < 0.05); c, significant difference for SPIR compared with CHLO (P < 0.05). CHLO, chlorella; iAUC, incremental area under the curve; EAA, essential amino acids; MYCO, mycoprotein; NEAA, nonessential amino acids; SPIR, spirulina; TAA, total amino acids.
Postprandial peak plasma NEAA concentrations were greater in SPIR than in CHLO (P < 0.05), although the overall increase in plasma NEAA concentrations over the 4-h postprandial period did not differ between groups (P = 0.10). Postabsorptive plasma leucine concentrations increased and remained elevated for the entire postprandial period following drink ingestion (time effect; P < 0.001), but to varying extents between groups (time × group interaction; P < 0.001). Plasma peak leucine concentrations were higher following SPIR ingestion than MYCO or CHLO ingestion, with peak values of 316 ± 66, 226 ± 30, and 187 ± 30 μmol·L-1, respectively (P < 0.001). Also, plasma leucine concentrations remained higher after SPIR ingestion than MYCO or CHLO ingestion at 15–60 min and 15–90 min, respectively (both P < 0.01). Postprandial plasma leucine concentrations were higher following MYCO ingestion than CHLO ingestion at 45–180 min (P < 0.05). Greater total plasma leucine availability over the postprandial period, as expressed by iAUC, was observed in SPIR compared with MYCO and CHLO groups (P < 0.05).
The remaining individual plasma amino acid responses are presented in Supplementary Figure 3. Similar responses were observed for all remaining individual plasma amino acid concentrations, with increases following protein ingestion (except for alanine) (time effects; all P < 0.001), and to differing degrees between groups (time × group interactions; all P < 0.01). Total plasma amino acid availability over the 4-h postprandial period was different between groups for isoleucine, lysine, methionine, phenylalanine, serine, threonine, and tyrosine (all P < 0.05).
Plasma and skeletal muscle tracer enrichments
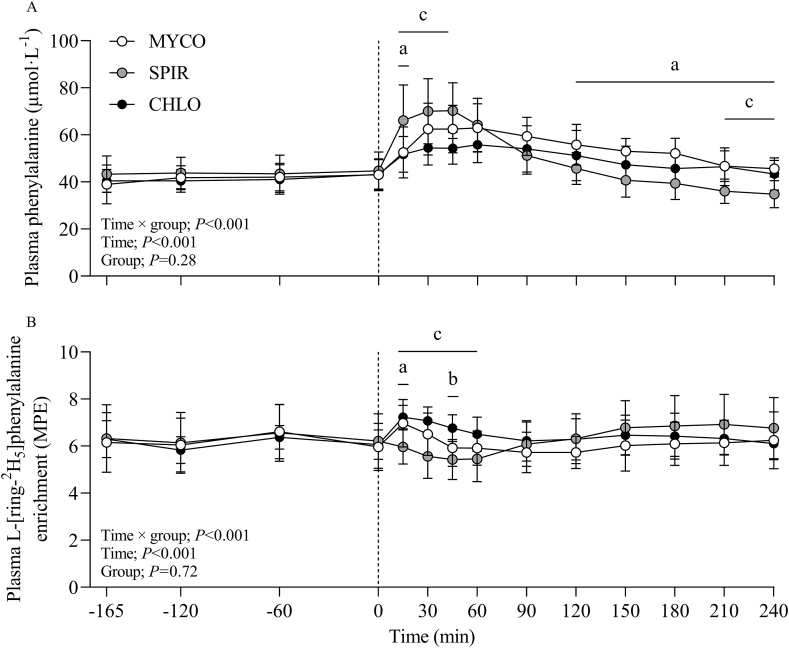
Plasma phenylalanine concentrations and L-[ring-2H5]-phenylalanine enrichments over time are presented in Figure 4. During the postabsorptive period, mean plasma L-[ring-2H5]-phenylalanine enrichments were stable at 6.2 ± 0.3, 6.3 ± 0.2, and 6.1 ± 0.2 mole percent excess in MYCO, SPIR, and CHLO, respectively, with no differences between groups (P = 0.69). Plasma L-[ring-2H5]-phenylalanine enrichments changed following drink ingestion (time effect; P < 0.001), and to a varying extent between groups (time × group interaction; P < 0.001). Specifically, plasma L-[ring-2H5]-phenylalanine enrichments increased for 15 and 45 min following MYCO and CHLO ingestion, respectively (P < 0.01), and decreased at 30–60 min postingestion of SPIR (P < 0.01). Plasma L-[ring-2H5]-phenylalanine enrichments were higher in CHLO than in MYCO at 45 min (P < 0.05) and SPIR at 15–60 min (P < 0.05), and were higher in MYCO than in SPIR at 15 min (P < 0.05). Weighted mean plasma L-[ring-2H5]-phenylalanine enrichments during the entire postprandial period were 6.1 ± 1.0, 6.4 ± 0.3, and 6.4 ± 0.6 mole percent excess in MYCO, SPIR, and CHLO, respectively (P = 0.43). Myofibrillar protein-bound L-[ring-2H5]-phenylalanine enrichment data are presented in the online Supplementary Data.
FIGURE 4.
Plasma phenylalanine concentration (A) and L-[ring-2H5]-phenylalanine enrichment (B) over time during the postabsorptive and 4-h postprandial period following the ingestion of 25 g protein from mycoprotein (MYCO; n = 12), spirulina (SPIR; n = 12) or chlorella (CHLO; n = 12) and execution of a bout of unilateral-resistance leg exercise in healthy young adults. Time 0 represents the time of protein ingestion. Values are mean ± SD. Data were analyzed with two-way repeated-measures ANOVA (time × group), with Bonferroni post hoc tests used to detect differences at individual intervals. Time × group interaction; both P < 0.001. a, significant difference for MYCO compared with SPIR (P < 0.05); b, significant difference for MYCO compared with CHLO (P < 0.05); c, significant difference for SPIR compared with CHLO (P < 0.05). CHLO, chlorella; MPE, mole percent excess; MYCO, mycoprotein; SPIR, spirulina.
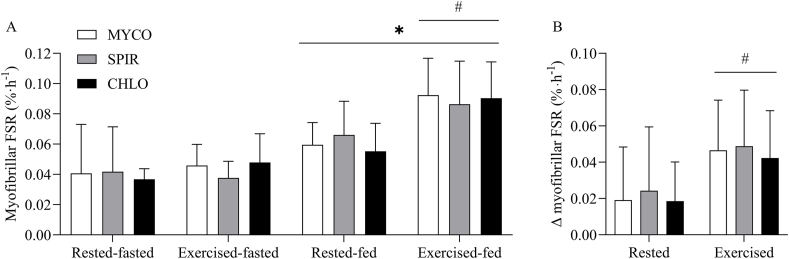
MyoPS
Myofibrillar FSRs calculated using the plasma precursor pool are depicted in Figure 5. Postabsorptive myofibrillar FSRs were similar between groups in both rested (MYCO, 0.041 ± 0.032; SPIR, 0.042 ± 0.030; CHLO, 0.037 ± 0.007%·h−1; P = 0.88) and exercised (MYCO, 0.046 ± 0.014; SPIR, 0.038 ± 0.011; CHLO, 0.048 ± 0.019%·h−1; P = 0.18) muscle, with no difference between legs (group × exercise interaction; P = 0.38). Drink ingestion increased myofibrillar FSRs in resting and exercised muscle in all groups (time effect; P < 0.001), but to a greater extent in exercised than in rested tissue (time × exercise interaction; P < 0.001). The increase in postprandial myofibrillar FSR was equivalent between groups in both rested and exercised muscle (time × group × exercise interaction; P = 0.96).
FIGURE 5.
Myofibrillar FSR calculated using the plasma L-[ring-2H5]-phenylalanine precursor pool during the postabsorptive (fasted) and postprandial (fed) period (A) and Δ FSR change from postabsorptive to postprandial state (B) for MYCO, SPIR, and CHLO conditions in rested and exercised (unilateral-resistance leg exercise) muscle in healthy young adults. The postprandial state represents the 4-h period following the ingestion of 25 g protein from mycoprotein (MYCO; n = 12), spirulina (SPIR; n = 12), or chlorella (CHLO; n = 12) and execution of a bout of unilateral-resistance leg exercise. Values are mean ± SD. Data were analyzed with three-way (time × group × exercise) ANOVA (P = 0.96). The Δ FSR data were analyzed with two-way (group × exercise) ANOVA (P = 0.96). ∗Significant difference between fasted and fed conditions (main effect of time; P < 0.001). #Significant difference between resting and exercised muscle in fed conditions (time × exercise interaction; P < 0.001). CHLO, chlorella; FSR, fractional synthetic rate; MYCO, mycoprotein; SPIR, spirulina.
In the rested leg, myofibrillar FSR increased from 0.041 ± 0.032 to 0.060 ± 0.015%·h−1, from 0.042 ± 0.030 to 0.066 ± 0.022%·h−1, and from 0.037 ± 0.007 to 0.055 ± 0.019%·h−1, following the ingestion of MYCO, SPIR, and CHLO, respectively. In the exercised leg, myofibrillar FSR increased from 0.046 ± 0.014 to 0.092 ± 0.024%·h−1, from 0.038 ± 0.011 to 0.086 ± 0.028%·h−1, and from 0.048 ± 0.019 to 0.090 ± 0.024%·h−1, in MYCO, SPIR, and CHLO, respectively. As a result, the delta increases from postabsorptive to postprandial myofibrillar FSRs were equivalent between groups in both resting (MYCO, Δ0.019 ± 0.029%·h−1; SPIR, Δ0.024 ± 0.035%·h−1; and CHLO, Δ0.019 ± 0.022%·h−1) and exercised (MYCO, Δ0.047 ± 0.028%·h−1; SPIR, Δ0.049 ± 0.031%·h−1; and CHLO, Δ0.042 ± 0.026%·h−1) muscle (group effect; P = 0.82), but were greater in the exercised than in the rested leg (exercise effect; P < 0.001).
Discussion
In this study, we assessed the MyoPS response to the ingestion of 2 novel protein- and EAA-rich algae and an isonitrogenous bolus of fungal-derived protein (MYCO) in healthy young adults. The ingestion of 25 g protein from SPIR, CHLO, and MYCO elicited strikingly divergent postprandial plasma amino acid responses.However, the consequent stimulations of both resting and postexercise MyoPS rates were similar among the food sources.
Ingestion of conventional protein-dense animal-derived foods is an established strategy for stimulating resting and postexercise MPS rates [9,[13], [14], [15], [16]]. Plant-based protein sources are generally considered of lesser anabolic quality due, at least partly, to incomplete or deficient amino acid profiles (e.g., low in leucine, lysine, or methionine) and/or inferior protein digestion and amino acid absorption kinetics [7,14,15]. We recently identified fungal-derived MYCO as a high-quality alternative dietary protein source capable of supporting muscle anabolism across various studies [[28], [29], [30],37]. Therefore, we reasoned that MYCO is a suitable control dietary protein for comparative purposes in studies seeking to identify other alternative sources capable of supporting muscle anabolism. Our present data replicate our previous findings by demonstrating that MYCO ingestion results in a relatively slow but sustained elevation of circulating amino acids (Figure 3), facilitating a robust (∼1.5 to 2-fold) stimulation of resting and postexercise MyoPS rates (Figure 5).
The present data (Table 2) confirm [18,41] that the commercially available cyanobacterium SPIR and microalgae CHLO are foods rich in protein (62% and 60% of total mass, respectively), EAA (40% and 41% of total protein), and leucine (10% and 9% of total protein), which is comparable to MYCO (and many protein-rich animal-based foods) [42], and have no deficiencies in amino acids according to FAO/WHO/UNU guidelines [43]. However, following ingestion, marked differences in postprandial aminoacidemia were observed (Figure 3; Supplementary Figure 3). SPIR protein appeared to be digested most rapidly, creating greater peak responses of most amino acids, regardless of initial content in the drink. In contrast, CHLO ingestion had a considerably slower and lower impact on plasma amino acid concentrations compared with SPIR or MYCO. Given that the 3 protein drinks were matched for total protein content, these findings illustrate how the amino acid content and composition of an ingested protein source do not solely explain observed postprandial plasma aminoacidemic responses. Greater carbohydrate [44,45], fat [46], fiber [47] contents, and other phytonutrients [48], have all been demonstrated to delay or impair gastric emptying, protein digestion, and/or intestinal amino acid absorption. Although the macronutrient profiles of the 3 experimental drinks were relatively similar (Table 2), fiber content was substantially higher in MYCO (11.3 g) than in SPIR (3.6 g) and CHLO (3.3 g), possibly explaining the delayed postprandial aminoacidemic response of this protein source. However, the slower and reduced postprandial availability of plasma amino acids following CHLO ingestion may be more related to the consumption of the food matrix, as opposed to consuming protein isolates or concentrates with or without the coingestion of other macronutrients. Contrary to SPIR/cyanobacteria [49], CHLO species, and more generally microalgae, exist within a complex multi-layered cell wall containing various insoluble and indigestible polysaccharides (predominantly glucosamine and cellulose) [50,51]. Although these polysaccharides provide structural stability and rigidity to the cell [51], they impair in vivo nutrient (including amino acid) bioavailability following CHLO ingestion. Although commercially available CHLO supplements, such as the one used in this study, have often undergone some degree of mechanical or chemical treatment to disrupt the cell walls [52,53], our data demonstrate that this may not be fully effective given the ∼63% and ∼79% lower total postprandial amino acid availability throughout the postprandial period compared with SPIR and MYCO, respectively.
We observed a robust increase in MyoPS rates in resting (∼1.5-fold) and exercised (∼2-fold) muscle in response to protein ingestion; however, contrary to our hypothesis, the increase was equivalent across all 3 protein sources. Therefore, despite the hypothesized divergent postprandial plasma amino acid responses coming to pass, this did not dictate differences in the magnitude of the MyoPS response. Previous work has repeatedly demonstrated an association between protein digestion or amino acid absorption kinetics and the subsequent MPS response [9,11,15,54], although such studies have typically used isolated or concentrated protein sources. The present data supplement a growing body of evidence suggesting a disassociation between the magnitude of plasma amino acid (e.g., leucine) responses and postprandial MPS rates following whole food ingestion either at rest or postexercise. For example, whole food ingestions, such as egg [13], beef [16], cheese [55], salmon [56], or MYCO [28,37], all elicited an attenuated postprandial amino acid response but equivalent or greater stimulations of MPS compared with isolated or more concentrated protein comparators.
The nature of whole foods compared with isolated protein sources generates additional variables beyond plasma amino acid kinetics that could have potentially modulated the anabolic response [57,58]. For example, the presence of dietary fiber in MYCO and CHLO [59,60], antioxidants and carotenoids in SPIR and CHLO [51,61] and/or large quantities of omega-3 PUFAs and vitamin D in CHLO [51] have all been linked with upregulating anabolic signaling pathways [[62], [63], [64], [65]], thus plausibly explaining our inability to detect differences in postprandial MyoPS rates between these diverse protein-rich foods. Alternatively, despite peak leucine concentrations being ∼19% and ∼56% lower following the ingestion of CHLO (initially providing 2.1 g leucine) compared with MYCO (1.7 g leucine) and SPIR (2.3 g leucine), it may still have been sufficient to exceed the threshold required to robustly increase MyoPS, or may have saturated the MyoPS response entirely [66]. If so, this would suggest that both the required amount of leucine for ingestion and, crucially, the required threshold for postprandial leucinemia, are considerably lower for optimally stimulating MyoPS than previously assumed based on dose-response studies using high-quality (animal-derived) isolated protein sources [66,67]. Future studies are warranted to assess the effect of ingesting smaller or suboptimal doses, and habitual, chronic algal consumption to underpin the role of the algal food matrix on skeletal muscle anabolism. Furthermore, the present findings cannot be translated directly to older and/or compromised adults displaying a reduced anabolic response to protein ingestion [68], in which even larger doses may be required for maximal MPS responses. Consequently, more studies will be needed to assess the anabolic properties of algal protein to mitigate the age-related decline in skeletal muscle quantity and quality.
The findings of the present work significantly add to the evidence base for the anabolic properties of nonanimal-derived protein sources, introducing novel data on a largely unexplored subclass of nonanimal-derived proteins. By demonstrating that algae are rich in protein and amino acids and can robustly stimulate resting and postexercise MyoPS, we provide proof-of-concept for algae as a novel alternative food source and strategy to support skeletal muscle maintenance and/or anabolism. To exploit these findings for societal impact, algal food production processes should focus on characterizing the anabolic potential of alternative protein sources, including various algal species, the impact of repeatedly ingesting different quantities of SPIR and CHLO on skeletal muscle remodeling, the possibilities of creating algal protein isolates, as well as optimizing algal digestibility and subsequent in vivo substrate availability. It also remains to be established whether consuming multiple large portions of algae is associated with potential side effects due to excessive micronutrient intake or palatability issues. Despite observing no differences in overall palatability between algae and MYCO, at least within our format (i.e., double-blinded, flavored drinks; Supplementary Table 1), algal foods are typically surrounded by food neophobia and unpleasant sensory characteristics (i.e., odor, taste, and texture) [69,70], likely requiring further optimization to increase palatability and consumer acceptance. Nevertheless, to our knowledge, the present study is the first to demonstrate algae as a viable protein source to support skeletal muscle anabolism and provides a strong incentive for algae to become a major protein source as part of a sustainable food future.
In conclusion, ingestion of protein-matched boluses of SPIR or CHLO robustly stimulates MyoPS in resting and exercised muscle tissue, and to an equivalent extent as a high-quality nonanimal-derived counterpart (MYCO). These findings identify algae as a viable alternative protein source to support postexercise skeletal muscle reconditioning in healthy young adults.
Author contributions
The authors’ contributions were as follows – IvdH, SW, AJM, FBS, BTW: designed the research; IvdH, FBS, BTW: conducted the research; IvdH DRA: analyzed the data; IvdH, BTW: performed the statistical analyses; IvdH, BTW: wrote the manuscript; BTW: took primary responsibility for the final content; SW: was responsible for randomization and preparation of the experimental beverages; and all authors: read and approved the final manuscript.
Conflicts of interest
TJAF was an employee of Marlow Foods; AJM, FBS, and BTW are employees of the University of Exeter; DRA and AJM are supported in part by a grant from the National Institute of Aging (P30-AG024832). All other authors report no conflicts of interest.
Funding
The authors report no funding received for this study.
Data availability
Data described in the manuscript may be made available upon request, pending application.
Acknowledgments
We acknowledge Premier Analytical Services for analyzing the nutritional composition of the experimental proteins.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.08.035.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 2.
Multimedia component 3.
Multimedia component 4.
References
- 1.Groen B.B., Horstman A.M., Hamer H.M., de Haan M., van Kranenburg J., Bierau J., et al. Post-prandial protein handling: you are what you just ate. PLoS. One. 2015;10(11) doi: 10.1371/journal.pone.0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips S.M., Tipton K.D., Aarsland A., Wolf S.E., Wolfe R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997;273(1 Pt 1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G., Tipton K.D., Klein S., Wolfe R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997;273(1 Pt 1):E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 4.Tipton K.D., Ferrando A.A., Phillips S.M., Doyle D., Jr., Wolfe R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 1999;276(4):E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 5.Volpi E., Kobayashi H., Sheffield-Moore M., Mittendorfer B., Wolfe R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003;78(2):250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita S., Dreyer H.C., Drummond M.J., Glynn E.L., Cadenas J.G., Yoshizawa F., et al. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007;582(Pt 2):813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vliet S., Burd N.A., van Loon L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015;145(9):1981–1991. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 8.Gorissen S.H.M., Crombag J.J.R., Senden J.M.G., Waterval W.A.H., Bierau J., Verdijk L.B., et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennings B., Boirie Y., Senden J.M., Gijsen A.P., Kuipers H., van Loon L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011;93(5):997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 10.Boirie Y., Dangin M., Gachon P., Vasson M.P., Maubois J.L., Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA. 1997;94(26):14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West D.W., Burd N.A., Coffey V.G., Baker S.K., Burke L.M., Hawley J.A., et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise1–4. Am. J. Clin. Nutr. 2011;94(3):795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 12.Churchward-Venne T.A., Snijders T., Linkens A.M., Hamer H.M., van Kranenburg J., van Loon L.J. Ingestion of casein in a milk matrix modulates dietary protein digestion and absorption kinetics but does not modulate postprandial muscle protein synthesis in older men. J. Nutr. 2015;145(7):1438–1445. doi: 10.3945/jn.115.213710. [DOI] [PubMed] [Google Scholar]
- 13.van Vliet S., Shy E.L., Abou Sawan S., Beals J.W., West D.W., Skinner S.K., et al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr. 2017;106(6):1401–1412. doi: 10.3945/ajcn.117.159855. [DOI] [PubMed] [Google Scholar]
- 14.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009;107(3):987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 15.Gorissen S.H., Horstman A.M., Franssen R., Crombag J.J., Langer H., Bierau J., et al. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 2016;146(9):1651–1659. doi: 10.3945/jn.116.231340. [DOI] [PubMed] [Google Scholar]
- 16.Burd N.A., Gorissen S.H., van Vliet S., Snijders T., van Loon L.J. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am. J. Clin. Nutr. 2015;102(4):828–836. doi: 10.3945/ajcn.114.103184. [DOI] [PubMed] [Google Scholar]
- 17.Searchinger T., Waite R., Hanson C., Ranganathan J., Dumas P., Matthews E., et al. World Resources Institute; 2019. Creating a sustainable food future: a menu of solutions to feed nearly 10 billion people by 2050.https://research.wri.org/sites/default/files/2019-07/WRR_Food_Full_Report_0.pdf Final report. Available from: [Google Scholar]
- 18.van der Heijden I., Monteyne A.J., Stephens F.B., Wall B.T. Alternative dietary protein sources to support healthy and active skeletal muscle aging. Nutr. Rev. 2023;81(2):206–230. doi: 10.1093/nutrit/nuac049. [DOI] [PubMed] [Google Scholar]
- 19.Aleksandrowicz L., Green R., Joy E.J., Smith P., Haines A. The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: a systematic review. PLoS. One. 2016;11(11) doi: 10.1371/journal.pone.0165797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finnigan T., Needham L., Abbott C. Elsevier; 2017. Mycoprotein: a healthy new protein with a low environmental impact, Sustainable protein sources; pp. 305–325. [DOI] [Google Scholar]
- 21.Klamczynska B., Mooney W.D. In: Sustainable protein sources. Nadathur S.R., Wanasundara J.P.D., Scanlin L., editors. Academic Press; San Diego: 2017. Chapter 20 - Heterotrophic microalgae: a scalable and sustainable protein source; pp. 327–339. [Google Scholar]
- 22.Clune S., Crossin E., Verghese K. Systematic review of greenhouse gas emissions for different fresh food categories. J. Clean. Prod. 2017;140:766–783. doi: 10.1016/j.jclepro.2016.04.082. [DOI] [Google Scholar]
- 23.Yang Y., Churchward-Venne T.A., Burd N.A., Breen L., Tarnopolsky M.A., Phillips S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. (Lond). 2012;9(1):57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinckaers P.J.M., Kouw I.W.K., Hendriks F.K., van Kranenburg J.M.X., de Groot L., Verdijk L.B., et al. No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br. J. Nutr. 2021;126(12):1832–1842. doi: 10.1017/s0007114521000635. [DOI] [PubMed] [Google Scholar]
- 25.Pinckaers P.J.M., Hendriks F.K., Hermans W.J.H., Goessens J.P.B., Senden J.M., van Kranenburg J.M.X., et al. Potato protein ingestion increases muscle protein synthesis rates at rest and during recovery from exercise in humans. Med. Sci. Sports Exerc. 2022;54(9):1572–1581. doi: 10.1249/mss.0000000000002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinckaers P.J.M., Kouw I.W.K., Gorissen S.H.M., Houben L.H.P., Senden J.M., Wodzig W.K.H.W., et al. The muscle protein synthetic response to the ingestion of a plant-derived protein blend does not differ from an equivalent amount of milk protein in healthy young males. J. Nutr. 2023;152(12):2734–2743. doi: 10.1093/jn/nxac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouw I.W.K., Pinckaers P.J.M., Le Bourgot C., van Kranenburg J.M.X., Zorenc A.H., de Groot L.C.P.G.M., et al. Ingestion of an ample amount of meat substitute based on a lysine-enriched, plant-based protein blend stimulates postprandial muscle protein synthesis to a similar extent as an isonitrogenous amount of chicken in healthy, young men. Br. J. Nutr. 2021;9:1–11. doi: 10.1017/s0007114521004906. [DOI] [PubMed] [Google Scholar]
- 28.Monteyne A.J., Coelho M.O.C., Porter C., Abdelrahman D.R., Jameson T.S.O., Jackman S.R., et al. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: a randomized controlled trial. Am. J. Clin. Nutr. 2020;112(2):318–333. doi: 10.1093/ajcn/nqaa092. [DOI] [PubMed] [Google Scholar]
- 29.Monteyne A.J., Dunlop M.V., Machin D.J., Coelho M.O.C., Pavis G.F., Porter C., et al. A mycoprotein-based high-protein vegan diet supports equivalent daily myofibrillar protein synthesis rates compared with an isonitrogenous omnivorous diet in older adults: a randomized controlled trial. Br. J. Nutr. 2021;126(5):674–684. doi: 10.1017/S0007114520004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteyne A.J., Coelho M.O.C., Murton A.J., Abdelrahman D.R., Blackwell J.R., Koscien C.P., et al. Vegan and omnivorous high protein diets support comparable daily myofibrillar protein synthesis rates and skeletal muscle hypertrophy in young adults. J. Nutr. 2023;153(6):1680–1695. doi: 10.1016/j.tjnut.2023.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan M.I., Shin J.H., Kim J.D. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories. 2018;17(1):36. doi: 10.1186/s12934-018-0879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abstracts from the 2022 International Sport + Exercise Nutrition Conference. Int. J. Sport Nutr. Exerc. Metab. 2023;33(S1):S1–S17. doi: 10.1123/ijsnem.2022-0267. [DOI] [PubMed] [Google Scholar]
- 33.Oosthuyse T., Bosch A.N. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010;40(3):207–227. doi: 10.2165/11317090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Abumrad N.N., Rabin D., Diamond M.P., Lacy W.W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 35.Flint A., Raben A., Blundell J.E., Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 36.Niewiadomski W., Laskowska D., Gąsiorowska A., Cybulski G., Strasz A., Langfort J. Determination and prediction of one repetition maximum (1RM): safety considerations. J. Hum. Kinet. 2008;19(1):109–120. doi: 10.2478/v10078-008-0008-8. [DOI] [Google Scholar]
- 37.West S., Monteyne A.J., Whelehan G., Abdelrahman D.R., Murton A.J., Finnigan T.J.A., et al. Mycoprotein ingestion within or without its wholefood matrix results in equivalent stimulation of myofibrillar protein synthesis rates in resting and exercised muscle of young men. Br. J. Nutr. 2023;130(1):20–32. doi: 10.1017/s0007114522003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe R.R., Chinkes D.L. John Wiley & Sons; 2004. Isotope tracers in metabolic research: principles and practice of kinetic analysis. [Google Scholar]
- 39.West S., Monteyne A.J., Whelehan G., van der Heijden I., Abdelrahman D.R., Murton A.J., et al. Ingestion of mycoprotein, pea protein, and their blend support comparable post-exercise myofibrillar protein synthesis rates in resistance trained individuals. Am. J. Physiol. Endocrinol. Metab. 2023;325(3):E267–E279. doi: 10.1152/ajpendo.00166.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabielski P., Ford G.C., Persson X.M., Jaleel A., Dewey J.D., Nair K.S. Comparison of different mass spectrometry techniques in the measurement of L-[ring-(13)C6]phenylalanine incorporation into mixed muscle proteins. J. Mass Spectrom. 2013;48(2):269–275. doi: 10.1002/jms.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker E.W. Micro-algae as a source of protein, Biotechnol. Adv. 2007;25(2):207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.USDA, Agricultural Research Service . 2019. FoodData Central.https://fdc.nal.usda.gov/ [Internet] [Date accessed: June 2023]. Available from: [Google Scholar]
- 43.World Health Organization, United Nations University . World Health Organization; Washington, DC: 2007. Protein and amino acid requirements in human nutrition.https://iris.who.int/bitstream/handle/10665/43411/WHO_TRS_935_eng.pdf [Internet] [Date accessed: June 2023]. Available from: [PubMed] [Google Scholar]
- 44.Gorissen S.H., Burd N.A., Hamer H.M., Gijsen A.P., Groen B.B., van Loon L.J. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin. Endocrinol. Metab. 2014;99(6):2250–2258. doi: 10.1210/jc.2013-3970. [DOI] [PubMed] [Google Scholar]
- 45.Hamer H.M., Wall B.T., Kiskini A., de Lange A., Groen B.B., Bakker J.A., et al. Carbohydrate co-ingestion with protein does not further augment post-prandial muscle protein accretion in older men. Nutr. Metab. (Lond). 2013;10(1):15. doi: 10.1186/1743-7075-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorissen S.H.M., Burd N.A., Kramer I.F., van Kranenburg J., Gijsen A.P., Rooyackers O., et al. Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin. Nutr. 2017;36(2):429–437. doi: 10.1016/j.clnu.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Dunlop M.V., Kilroe S.P., Bowtell J.L., Finnigan T.J.A., Salmon D.L., Wall B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: a dose-response study. Br. J. Nutr. 2017;118(9):673–685. doi: 10.1017/s0007114517002409. [DOI] [PubMed] [Google Scholar]
- 48.Sarwar Gilani G., Wu Xiao C., Cockell K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012;108(Suppl_2):S315–S332. doi: 10.1017/s0007114512002371. [DOI] [PubMed] [Google Scholar]
- 49.Van Eykelenburg C. On the morphology and ultrastructure of the cell wall of Spirulina platensis. Antonie Leeuwenhoek. 1977;43(2):89–99. doi: 10.1007/BF00395664. [DOI] [PubMed] [Google Scholar]
- 50.Safi C., Zebib B., Merah O., Pontalier P.-Y., Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew. Sustain. Energ. Rev. 2014;35:265–278. doi: 10.1016/j.rser.2014.04.007. [DOI] [Google Scholar]
- 51.Bito T., Okumura E., Fujishima M., Watanabe F. Potential of chlorella as a dietary supplement to promote human health. Nutrients. 2020;12(9):2524. doi: 10.3390/nu12092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phong W.N., Show P.L., Ling T.C., Juan J.C., Ng E.P., Chang J.S. Mild cell disruption methods for bio-functional proteins recovery from microalgae—recent developments and future perspectives. Algal Res. 2018;31:506–516. doi: 10.1016/j.algal.2017.04.005. [DOI] [Google Scholar]
- 53.Weber S., Grande P.M., Blank L.M., Klose H. Insights into cell wall disintegration of Chlorella vulgaris. PLoS. One. 2022;17(1) doi: 10.1371/journal.pone.0262500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koopman R., Crombach N., Gijsen A.P., Walrand S., Fauquant J., Kies A.K., et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009;90(1):106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- 55.Hermans W.J.H., Fuchs C.J., Hendriks F.K., Houben L.H.P., Senden J.M., Verdijk L.B., et al. Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: a randomized parallel-group trial. J. Nutr. 2022;152(4):1022–1030. doi: 10.1093/jn/nxac007. [DOI] [PubMed] [Google Scholar]
- 56.Paulussen K.J., Barnes T.M., Askow A.T., Salvador A.F., McKenna C.F., Scaroni S.E., et al. Underpinning the food matrix regulation of postexercise myofibrillar protein synthesis by comparing salmon ingestion with the sum of its isolated nutrients in healthy young adults. J. Nutr. 2023;153(5):1359–1372. doi: 10.1016/j.tjnut.2023.02.037. [DOI] [PubMed] [Google Scholar]
- 57.Burd N.A., Beals J.W., Martinez I.G., Salvador A.F., Skinner S.K. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Med. 2019;49(Suppl 1):59–68. doi: 10.1007/s40279-018-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vliet S.V., Beals J.W., Martinez I.G., Skinner S.K., Burd N.A. Achieving optimal post-exercise muscle protein remodeling in physically active adults through whole food consumption. Nutrients. 2018;10(2):224. doi: 10.3390/nu10020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finnigan T. 2011. Mycoprotein: origins, production and properties, Handbook of food proteins; pp. 335–352. [DOI] [Google Scholar]
- 60.Panahi Y., Darvishi B., Jowzi N., Beiraghdar F., Sahebkar A. Chlorella vulgaris: a multifunctional dietary supplement with diverse medicinal properties. Curr. Pharm. Des. 2016;22(2):164–173. doi: 10.2174/1381612822666151112145226. [DOI] [PubMed] [Google Scholar]
- 61.Wu Q., Liu L., Miron A., Klímová B., Wan D., Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016;90(8):1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 62.Smith G.I., Atherton P., Reeds D.N., Mohammed B.S., Rankin D., Rennie M.J., et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. (Lond). 2011;121(6):267–278. doi: 10.1042/cs20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salles J., Chanet A., Giraudet C., Patrac V., Pierre P., Jourdan M., et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol. Nutr. Food Res. 2013;57(12):2137–2146. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- 64.Marzani B., Balage M., Vénien A., Astruc T., Papet I., Dardevet D., et al. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats1,2. J. Nutr. 2008;138(11):2205–2211. doi: 10.3945/jn.108.094029. [DOI] [PubMed] [Google Scholar]
- 65.Kitakaze T., Harada N., Imagita H., Yamaji R. β-carotene increases muscle mass and hypertrophy in the soleus muscle in mice. J. Nutr. Sci. Vitaminol. (Tokyo). 2015;61(6):481–487. doi: 10.3177/jnsv.61.481. [DOI] [PubMed] [Google Scholar]
- 66.Moore D.R., Robinson M.J., Fry J.L., Tang J.E., Glover E.I., Wilkinson S.B., et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009;89(1):161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 67.Witard O.C., Jackman S.R., Breen L., Smith K., Selby A., Tipton K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014;99(1):86–95. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 68.Wall B.T., Gorissen S.H., Pennings B., Koopman R., Groen B.B., Verdijk L.B., et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS. One. 2015;10(11) doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onwezen M.C., Bouwman E.P., Reinders M.J., Dagevos H. A systematic review on consumer acceptance of alternative proteins: pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite. 2021;159:105058. doi: 10.1016/j.appet.2020.105058. [DOI] [PubMed] [Google Scholar]
- 70.Weinrich R., Elshiewy O. Preference and willingness to pay for meat substitutes based on micro-algae. Appetite. 2019;142:104353. doi: 10.1016/j.appet.2019.104353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript may be made available upon request, pending application.