Highlights
-
•
An 8-day naturally lacto-fermented cucumber product was developed for use.
-
•
Antiinflammatory characteristics of the product were assessed using RAW 264.7 cells.
-
•
Both water and ethanol extracts from the product inhibited LPS-induced inflammation.
-
•
Phenolic contents in sample were significantly correlated with IL-10 secretions.
Keywords: Anti-inflammation, Lipopolysaccharide, Naturally lacto-fermented cucumber, Pro-/anti-inflammatory cytokines, RAW 264.7 cells
Abstract
A naturally lacto-fermented cucumber product was developed for use as anti-inflammatory functional foods. To explore the anti-inflammatory characteristics, water (CWE) and ethanol extracts (CEE) from this product were selected to assess their anti-inflammatory potential on RAW 264.7 macrophages in the absence or presence of lipopolysaccharide (LPS), using four different inflammatory models. Changes in pro- (IL-1β, IL-6 and TNF-α) and anti-inflammatory (IL-10) cytokine secretions by treated macrophages were measured using ELISA. The results showed that both CWE and CEE had strong potential to inhibit LPS-stimulated inflammation in macrophages in a repair manner. CWE had a better effect than CEE. The total phenolic, flavonoid and saponin contents in CEE were significantly (P < 0.05) correlated with IL-10 (r = 0.384, P = 0.036*) and TNF-α (r = 0.371, P = 0.043*) levels, but slightly correlated with TNF-α/IL-10 secretion ratios (r = −0.184, P = 0.359) by treated RAW 264.7 cells, respectively.
Introduction
Fermentation that preserves food and enhances flavor, originating from traditional food processing methods is a vital part of the food industry (Sanlier, Gokcen, & Sezgin, 2019). However, fermented foods still have slight risks from their consumption resulting from contaminated pathogens, such as Escherichia coli, Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Salmonella spp. and Shigella spp., particularly in Africa and Asia (Skowron et al., 2022). Moreover, harmful components, including biogenic amines and nitrites produced by detrimental bacteria, may occur during the fermentation process (Dala-Paula et al., 2021, Zhao et al., 2021). Fortunately, most fermented foods have been found to possess health-promoting benefits attributed to diverse functional microorganisms such as probiotic lactic acid bacteria (LAB). Prebiotic ingredients, including exopolysaccharides, bioactive peptides, vitamins, minerals, conjugated linoleic acids (CLA) etc. are present in fermented foodstuffs, especially in plant-based fermented foods (Chourasia et al., 2023, Sanlier et al., 2019, Skowron et al., 2022, Wuyts et al., 2020). In recent years, minimally processed fermented foods with precision probiotics have been a beneficial nutritional and functional strategy that has gained popularity across the world (Diez-Ozaeta & Astiazaran, 2022).
Health-promoting benefits from fermented foods may emerge as anti-inflammatory, anti-microbial, anti-fungal, anti-oxidant, anti-diabetic, anti-allergenic, opioid antagonist, anti-atherosclerotic, anti-carcinogenic, anti-hypertensive, and immunomodulatory effects (Bhatia et al., 2023, Chourasia et al., 2023, Sanlier et al., 2019, Behera et al., 2020). Today, most chronic degenerative disorders, including chronic inflammatory pain, oxidative stress, osteoarthritis, and rheumatoid arthritis, are associated with long-term excess chronic inflammation in the body (Paul et al., 2023). Fermented foods containing probiotic LAB, fungi and prebiotic ingredients prepared from vegetables such as soy-based foods, sauerkraut, kimchi, or turmeric have been suggested to improve gastrointestinal health and the immune system. These foods may decrease the risk from several different inflammatory diseases (Paul et al., 2023). Limited clinical and translational experimental studies have been performed for assessing the effectiveness of various fermented foods against inflammation (Paul et al., 2023). To develop anti-inflammatory fermented foods for various health-promoting objectives, the claimed functional characteristics should be analyzed and evidenced (Sanlier et al., 2019, Behera et al., 2020).
Among various vegetables, fresh cucumber (Cucumis sativus L.) fruit with various bioactive phytochemicals, but without any apparent adverse side effects, has been reported to have anti-oxidant, anti-inflammatory, anti-microbial, anti-carcinogenic and anti-diabetic potential (Agatemor et al., 2015, Sharma et al., 2020). Fermented cucumber may further enhance its original health benefits through producing prebiotics, probiotics, symbiotic and/or postbiotics during the fermentation process (Zieliński, Surma, & Zielinska, 2017). Recently, a two-step trial has been designed to optimize culture conditions for developing functional naturally lacto-fermented cucumbers (Kao & Lin, 2023a). Based on changes in pH values, lactic acid, organic acids, total phenolic, flavonoid, saponin, and free amino acid levels, as well as total biogenic amines and nitrites during fermentation process, an 8-day naturally lacto-fermented cucumber product using a 15 % crystal sugar solution with 2.5 % low-salt brine pickling formula at 4 °C has been developed (Kao & Lin, 2023a). In addition, the sensory quality of the developed naturally lacto-fermented cucumber product was improved, and total LAB counts in the brine increased time-dependently while the dominant LAB species was identified as Leuconostoc mensenteroides (80.72 %) (Kao et al., 2023). The probiotic lactic acid bacteria fermentation may increase functional phenolic acids and consequently enhance anti-oxidative activities (Li et al., 2024). In addition, diverse functional food components in the developed fermented cucumbers, such as active peptides, free amino acids, organic acids, oligosaccharides, exopolysaccharides, and vitamins may increase, but detrimental microbes, oxidants, and hypersensitivities may decrease and eliminate using innovative fermentation processing technologies (Liu, Wang, & Deng, 2023). However, the anti-inflammatory characteristics of the developed naturally lacto-fermented cucumber product remain unknown.
To explore the anti-inflammatory characteristics, extracts of the developed naturally lacto-fermented cucumber product were assessed using RAW 264.7 macrophages under four different inflammatory models in the absence or presence of lipopolysaccharide (LPS). Changes in pro- (IL-1β, IL-6, TNF-α)/anti-inflammatory (IL-10) cytokines produced by treated RAW 264.7 macrophages were measured with an enzyme-linked immunosorbent assay (ELISA) (Kao & Lin, 2023b). The relationships between the functional ingredient contents in the extract product and anti-inflammatory effects were analyzed using the Pearson’s product-moment correlation coefficient (r).
Materials and methods
Materials
Fresh immature cucumber (Cucumis sativus L.) fruits were bought from a local supermarket in Taichung City, Taiwan. The fruits were washed with tap water, dripped dry, and sliced into 3–5 cm long quadrants for fermentation (Kao et al., 2023, Kao and Lin, 2023a).
Naturally lacto-fermented cucumber preparation under the established optimal pickling juice formula (15 % crystal sugar solution with brine)
In our preliminary study, an experiment with a two-step trial was performed to optimize the naturally developed lacto-fermented cucumber product. A 15 % crystal sugar solution with 2.5 % low-salt brine at 4 °C was evidenced to be the best pickling formula according to pH values and total biogenic amines in the first trial (Kao & Lin, 2023a). The optimal fermentation day was proved at around 8 days in the second trial based on changes in pH values, organic acids, free amino acids, total phenolics, flavonoids, saponins, biogenic amines and nitrites using the established optimal pickling formula, confirming functional characteristics and the safety for the 8-day naturally lacto-fermented cucumbers (Kao & Lin, 2023a). Through the optimal fermentation process both sensory profile and color difference in the 8-day lacto-fermented cucumber product significantly improved. Meanwhile, lactic acid bacteria count time-dependently and significantly increased through the 8-day aging process; Leuconostoc mensenteroides were evidenced as the dominant (80.72 %) lactic acid bacteria (Kao et al., 2023). Based on the overall preliminary evaluation, the optimal pickling juice formula (15 % crystal sugar solution with 2.5 % NaCl) at 4 °C for 8 days was adopted to prepare the naturally lacto-fermented cucumber product in this study (Kao et al., 2023, Kao and Lin, 2023a). Briefly, aliquots of 200 g of cucumbers were added with 5 g of salt (NaCl, 2.5 %, w/w), mixed thoroughly, and stood at room temperature for 1 h for dehydration. Water drawn out from the cucumbers and salt (brine) were preserved with the cucumbers and collected into a 500 ml fermented glass can. Aliquots of 150 ml 15 % crystal sugar solution were added to the containers to cover the cucumbers, thoroughly mixed and the lids opened for exposure to environmental airborne microorganisms for 30 min. After the natural exposure, the lids were screwed tight and the containers were naturally fermented in a 4 °C refrigerator for 8 days. During the fermentation period, changes in the appearance and divergent chemical parameters were observed to monitor the safety and quality (Kao et al., 2023, Kao and Lin, 2023a). The aged 8-day naturally lacto-fermented cucumbers harvested from different batch processing were pooled for analyzing possible anti-inflammatory potential using RAW 264.7 macrophages with four different inflammatory models (Kao & Lin, 2023b). A commercial kimchi product was also selected for comparison.
Extracts preparation of the naturally lacto-fermented cucumber product and a commercial kimchi product
The 8-day naturally lacto-fermented cucumbers were harvested and a commercial kimchi product was bought to compare possible anti-inflammatory potential. Both samples were weighted and lyophilized for 72 h. The lyophilized samples were weighted, ground into powder and stored at 4 °C until use. To evaluate the anti-inflammatory potential, water and ethanol extracts from samples were isolated for assay, respectively (Kao & Lin, 2023b).
Water extract preparation
Briefly, an aliquot of 50 g lyophilized powder sample was weighed, added with 500 ml deionized water and gently stirred for 6 h at room temperature to prepare the water extract. The mixture was centrifuged at 600 × g for 15 min at 25 °C using a centrifuge machine (automatic high speed refrigerated centrifuge, Hitachi, CR20B2, Tokyo, Japan). The supernatant was carefully harvested and evaporated to concentrate the water extract using a vacuum evaporator (EYELA rotary vacuum evaporator, Tokyo Rikakika Co., N-1200AVF, Japan) aided with an aspirator (EYELA Aspirator, Tokyo Rikakika Co., A-1000S, Japan). The extract was lyophilized into powder for 72 h using a freeze dryer. The lyophilized powder (water extract) was weighed to compute the water extract yield (%, w/w). Water extracts from the 8-day naturally lacto-fermented cucumber (CWE) and commercial kimchi product (KWE) were weighed and re-dissolved in deionized water to achieve a fixed concentration of 100 mg/ml as a stock solution, respectively. The water extract stock solution was sterilized using a 0.22 μm pore size filter (Merck Millipore Ltd., Tullagreen Carrigtwohill Co., Cork, IRL) and stored at −30 °C until use. Working solutions at appropriate concentrations diluted with cell culture media were prepared when used (Kao & Lin, 2023b).
Ethanol extract preparation
Briefly, an aliquot of 50 g lyophilized powder sample was weighed, added with 250 ml 95 % ethanol, and gently stirred for 4 h at room temperature to prepare the ethanol extract. The mixture was centrifuged at 600×g at 25 °C for 10 min using a centrifuge machine (automatic high speed refrigerated centrifuge, Hitachi, CR20B2, Tokyo, Japan). The supernatant was carefully harvested and the solvent completely removed using a vacuum evaporator (EYELA rotary vacuum evaporator, Tokyo Rikakika Co., N-1200AVF, Japan) aided by an aspirator (EYELA Aspirator, Tokyo Rikakika Co., A-1000S, Japan). The residue (ethanol extract) was weighed to compute the ethanol extract yield (%, w/w). Total phenolic, flavonoid and saponin amounts in the ethanol extracts of fermented cucumbers were measured (Kao and Lin, 2023a, Zhang et al., 2023). Ethanol extracts from the 8-day naturally lacto-fermented cucumber (CEE) and commercial kimchi product (KEE) were weighed and re-dissolved in 95 % ethanol to achieve a fixed concentration of 25 mg/ml as a stock solution, respectively. The ethanol extract stock solution was sterilized using a 0.22 μm pore size filter (Acrodisc Syringe Filters, DMSO-Safe, Sterile, Pall Life Sciences) and stored at −30 °C until use. Working solutions at appropriate concentrations diluted with cell culture media were prepared when used (Kao & Lin, 2023b).
Anti-inflammatory potential evaluation of extracts from the naturally lacto-fermented cucumber product and a commercial kimchi product using RAW 264.7 macrophages in vitro
RAW 264.7 cell cultures
The RAW 264.7 cell line (BCRC No. 60001) was originated from a murine BALB/c macrophage and transformed using the Abelson murine leukemia virus was provided by the Bioresource Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (FIRDI), Hsinchu, Taiwan. The frozen RAW 264.7 cell line was directly defrosted using a 37 °C water bath and immediately cultured in a 10 cm petri dish (100 × 20 mm) with 10 ml of Dulbecco's modified Eagle's medium (DMEM), composed of 1 L of DMEM (Hyclone, SH30022, Logan, UT, USA), 100 ml of 10 % fetal bovine serum (FBS, Biological Industries, 04-001A, Kibbutz Beit Haemek, Israel) and 5 ml Antibiotic-Antimycotic solution (100 × PSA, Corning, 30–004-C1, Mediatech, Inc., VA20109 Manassas, VA, USA) which contains 10,000 IU/ml Penicillin, 10,000 μg/ml Streptomycin and 25 µg/ml Amphotericin in 0.85 % NaCl solution. The defrosted cells were quickly cultured at 37 °C for 4 h in a humidified incubator with 5 % CO2 and 95 % air to allow the attachment of healthy adherent cells. The supernatant was carefully removed and healthy adherent RAW 264.7 cells were added with 10 ml fresh DMEM medium. The cells were maintained in an incubator with 5 % CO2 and 95 % air at 37 °C before growing to 90 % confluence. The cell culture supernatant was carefully removed, and the adherent cells were washed twice using 10 ml of sterile phosphate-buffered saline (1 × PBS, 137 mmol/l NaCl, 2.7 mmol/l KCl, 8.1 mmol/l Na2HPO4, 1.5 mmol/l KH2PO4, pH 7.4, 0.20 μm filtered). The adherent cells were added with an aliquot of 1 ml DMEM medium and harvested with a cell scraper (Corning Incorporated COSTAR 3008, Corning, NY, USA). The harvested cells were appropriately diluted at 1:3 to 1:6 split ratio with DMEM medium for subculture. When the cells had grown to 90 % confluence, the subculture process was repeated to harvest enough cell numbers for the following experiments. The harvested RAW 264.7 cells were adjusted to 2 × 105 cells/ml DMEM medium using the trypan blue (Sigma, T-78154, St. Louis, MO, USA) dye exclusion method. The RAW 264.7 cell line at appropriate passage numbers was adopted to perform the bioassay in vitro (Kao & Lin, 2023b).
Determination of optimal extract concentrations from the naturally lacto-fermented cucumber product and a commercial kimchi product using RAW 264.7 cells
To obtain optimal non-cytotoxic test sample concentrations for RAW 264.7 cells in vitro, water (0–1000 μg/ml) and ethanol extracts (0 – 100 μg/ml) were respectively selected to treat RAW 264.7 cells. Briefly, aliquots of 50 μl/well RAW 264.7 cells (2 × 105 cells/ml DMEM medium) were treated with aliquots of 50 μl/well extracts, 1 % solvent or lipopolysaccharide (LPS, a positive control, Sigma, L-2654, St. Louis, MO, USA) at a final concentration of 2.5 μg/ml in a 96-well plate. The plate was incubated in a humidified incubator at 37 °C with 5 % CO2 and 95 % air for 72 h. Treated RAW 264.7 cell viabilities were determined using 3-(4,5-dimethylthiazol-2-diphenyl)-2,5-tetrazolium bromide (MTT, Sigma M−5655, Missouri, USA) assay (Lin & Lin, 2011). Aliquots of 10 μl 5 mg/ml MTT in PBS were pipetted into the wells in the 96-well plate. The plate was cultured in the humidified incubator for another 4 h. The cell culture supernatant was carefully discarded after the plate was centrifuged at 25 °C, 400 × g for 10 min. The plate was washed with PBS buffer thrice. An aliquot of 100 μl dimethyl sulfoxide (DMSO) was pipetted into each well in the plate. The plate was gently shaken for 30 min to extract formed insoluble formazan which is produced by succinate dehydrogenase in the mitochondria of viable cells. The absorbance (A) at 550 nm was then measured with an ELISA reader (Microplate reader, FLUOstar-Omega, 415–1103, Germany). The cell viability was displayed as changes in cell numbers (% of control) and computed in comparison to the mean control absorbency with the following equation: changes in cell numbers (% of control) = [(Asample - Ablank)/(Acontrol - Ablank)] × 100 (Yeh & Lin, 2020). The non-cytotoxic optimal extract doses from the 8-day naturally lacto-fermented cucumbers and a commercial kimchi product to RAW 264.7 cells were selected to perform anti-inflammation assessments using four experimental models in vitro (Kao and Lin, 2023b, Lin and Lin, 2011).
Anti-inflammatory assessments of extracts from the naturally lacto-fermented cucumber product and a commercial kimchi product in vitro
To compare the anti-inflammatory potential of water and ethanol extracts from 8-day naturally lacto-fermented cucumbers (CWE and CEE) and a commercial kimchi product (KWE and KEE), samples at non-cytotoxic optimal doses were selected to treat RAW 264.7 macrophages in the absence or presence of LPS under four in vitro models (Kao and Lin, 2023b, Lin and Lin, 2011).
Model A: Test sample direct action on RAW 264.7 cells in the absence of LPS
To assess the test sample effects treated alone on RAW 264.7 cells, RAW 264.7 cells in the absence or presence of test samples at different non-cytotoxic optimal concentrations were co-cultured in 24-well plates for 24 h. Briefly, RAW 264.7 cells (2 × 105 cells/ml, 1.0 ml/well) were added to the wells in a 24-well plate and the plate was incubated for 3 h at 37 °C in an incubator with 5 % CO2 and 95 % air to allow the cells to attach. After the cell culture supernatant was removed, the attached RAW 264.7 cells in each well were added with an aliquot of 1.0 ml/well DMEM medium (control) and 1.0 ml/well samples in DMEM medium at their non-cytotoxic optimal concentrations and incubated for another 24 h. LPS alone (at 100 ng/ml) in each individual experiment was selected as a positive control. After the plate was centrifuged at 25 °C, 400 × g for 10 min, individual cell culture supernatant was collected and stored at −30 °C for pro-/anti-inflammatory cytokines assay (Kao & Lin, 2023b).
Model B: Simultaneous test sample effects on RAW 264.7 cells in the presence of LPS
To assess simultaneous effects of test samples on RAW 264.7 cells in the presence of LPS, RAW 264.7 cells (2 × 105 cells/ml, 1.0 ml/well) were respectively pipetted into each well in a 24-well plate. The plate was incubated at a 37 °C incubator for 3 h to allow intact adherent cells to attach and the cell culture supernatant was then carefully removed. Aliquots of 0.5 ml/well LPS (200 ng/ml), DMEM medium (control), 2 × test sample concentration at non-cytotoxic optimal concentrations and dexamethasone (DEX, 200 nM, as a treatment control) were added to the cells and incubated for 24 h. RAW 264.7 cells treated with DMEM alone were performed in each individual experiment as a naïve control. Finally, the plate was centrifuged at 25 °C, 400×g for 10 min to harvest the cell culture supernatants for cytokine assays (Kao & Lin, 2023b).
Model C: Test sample inflammatory repair effect on LPS-stimulated inflammation
To assess the repair (curative) effects of test samples on LPS-stimulated inflammation, RAW 264.7 cells (2 × 105 cells/ml, 1.0 ml/well) were pipetted into the wells in 24-well plates and incubated in an incubator at 37 °C for 3 h to allow intact adherent cells to attach. After the cell culture supernatant was carefully removed, the remaining attached RAW 264.7 cells in the 24-well plates were added with 1.0 ml/well LPS (100 ng/ml) for 12 h to induce inflammation in the cells. The plate was centrifuged at 400×g, 25 °C for 10 min to remove the cell culture supernatants. The RAW 264.7 cells were carefully washed twice with 1 × PBS. The LPS-treated RAW 264.7 cells were respectively added with 1.0 ml/well DMEM medium (control), test samples at non-cytotoxic optimal concentrations, and DEX (100 nM, as a treatment control) for another 12 h to repair the inflammation in the cells. RAW 264.7 cells in the absence of LPS post-treated with DMEM alone were performed in each individual experiment as a naïve control. Finally, the plate was centrifuged at 400×g, 25 °C for 10 min to harvest the cell culture supernatants for cytokine assays (Kao & Lin, 2023b).
Model D: Test sample inflammatory prophylactic effect on LPS-stimulated inflammation
To assess the prophylactic effects of test samples on LPS-stimulated inflammation, RAW 264.7 cells (2 × 105 cells/ml, 1.0 ml/well) were pipetted into the wells in 24-well plates and incubated in an incubator at 37 °C for 3 h to allow intact adherent cells to attach. After the cell culture supernatant was removed carefully, the remaining attached RAW 264.7 cells in the 24-well plates were respectively added with 1.0 ml/well DMEM medium (control), test samples at non-cytotoxic optimal concentrations, and DEX (100 nM, as a treatment control) and incubated for 12 h to induce the protective effects on the following LPS-stimulated inflammation. The plate was centrifuged at 400 × g, 25 °C for 10 min to remove the cell culture supernatants. The RAW 264.7 cells were washed twice with 1 × PBS. The sample-treated RAW 264.7 cells were further stimulated with 1.0 ml/well LPS (100 ng/ml) and incubated for another 12 h to induce inflammation. RAW 264.7 cells pre-treated with DMEM alone in the absence of LPS were performed in each individual experiment as a naïve control. Finally, the plate was centrifuged at 400×g, 25 °C for 10 min to harvest the cell culture supernatants for cytokine assays (Kao & Lin, 2023b).
Measurement of pro- and anti-inflammatory cytokine levels produced by treated RAW 264.7 cells with an enzyme-linked immunosorbent assay (ELISA)
The harvested RAW 264.7 cell culture supernatants in each individual treatment experiments were subjected to measure pro- (IL-1β, IL-6, and TNF-α) and anti-inflammatory (IL-10) cytokines levels using sandwich ELISA kits, respectively. The measurement of IL-6, TNF-α (mouse DuoSet ELISA Development system, R&D Systems, Minneapolis, MN, USA), IL-1β and IL-10 (mouse ELISA MAX Deluxe Set, Biolegend, San Diego, CA, USA) levels was performed based on the protocol provided by the manufacturer’s company, respectively (Yeh & Lin, 2020). The limit of detection (LOD) of IL-1β, IL-6, and IL-10 cytokines was < 15.6 pg/ml; TNF-α cytokine was < 31.2 pg/ml in this study.
Statistical analyses
Results are shown as means ± standard deviation (SD). All collected data were first examined with one-way analysis of variance (ANOVA) to check the homogeneity of variances, if justified by the computed probability (P < 0.05), followed by Duncan’s new multiple range test to perform post hoc analyses using IBM SPSS statistics version 20.0. Relationships between different bio-markers were assessed using the Pearson’s product moment correlation coefficient (r). P < 0.05 was statistically considered as significant differences among treatments.
Results and discussion
Optimal treatment concentrations of extracts from the naturally lacto-fermented cucumber product and a commercial kimchi product to RAW 264.7 cells
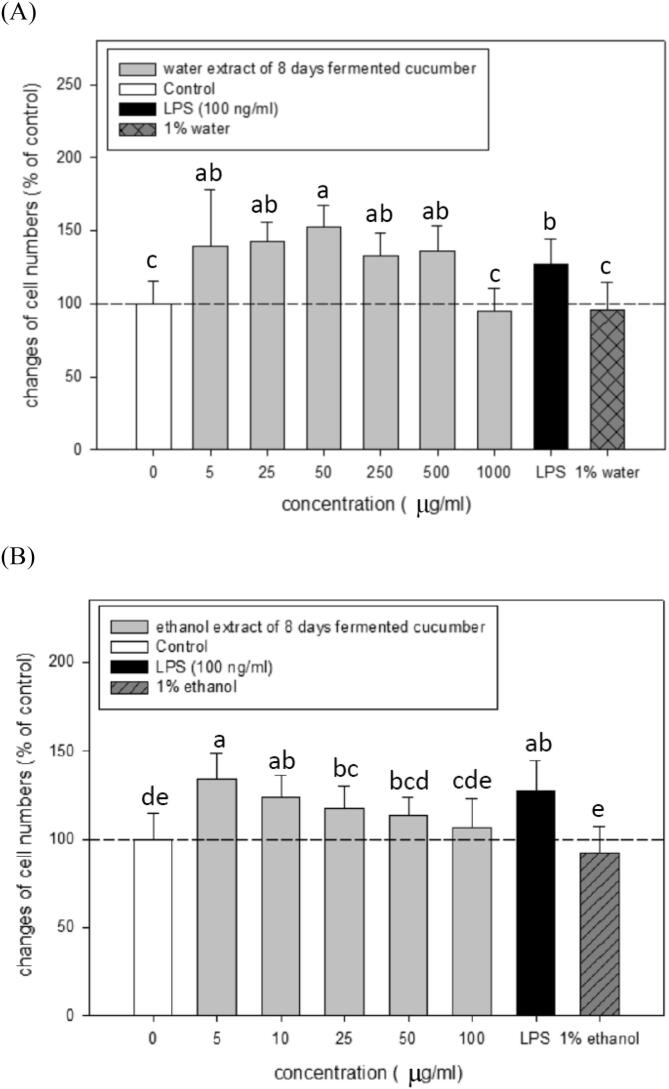
To obtain optimal non-cytotoxic concentrations for in vitro bioassay, the possible cyto-toxicities of water (CWE and KWE) and ethanol extracts (CEE and KEE) from the 8-day naturally lacto-fermented cucumber and commercial kimchi products at various concentrations were determined via treating RAW 264.7 cells for 72 h followed by a MTT assay. LPS (a positive control) at 100 ng/ml was found to significantly (P < 0.05) stimulate the proliferation and inflammation of RAW 264.7 cells compared to that of the control (Fig. 1 and Fig. 2). However, treatments of 1 % solvents (water and ethanol) alone that were the highest remaining percentage in each individual diluted samples did not significantly (P > 0.05) change RAW 264.7 cell viabilities compared to the controls, suggesting that the bioassay platform using RAW 264.7 cells is a suitable model for anti-inflammation analysis. Importantly, water and ethanol extracts from the 8-day naturally lacto-fermented cucumber (Fig. 1 (A, B)) and commercial kimchi (Fig. 1 (C, D)) at the indicated test concentrations did not cause obvious cyto-toxicities to RAW 264.7 macrophages, but significantly enhanced (P < 0.05) the RAW 264.7 cell proliferation at appropriate concentrations, respectively. Our results suggest that both water (CWE and KWE) and ethanol extracts (CEE and KEE) of the 8-day naturally lacto-fermented cucumber and commercial kimchi products at appropriate concentrations may have a stimulatory activity to RAW 264.7 macrophages. However, water extracts of both test products at 1,000 μg/ml still slightly decrease the cell viabilities compared to those of the controls (Fig. 1(A) and Fig. 1(C). To avoid an unpredicted test sample cytotoxicity at higher doses, appropriate lower concentrations should be adopted for further analyzing their anti-inflammatory potential using the following in vitro RAW 264.7 cell culture models. Based on the results (Fig. 1), the adopted suitable concentrations were 50, 250, and 500 μg/ml for water extracts (CWE and KWE); 10, 50, and 100 μg/ml for ethanol extracts (CEE and KEE), respectively. All of the adopted test concentrations did not inhibit the RAW 264.7 cell viability, suggesting that these adopted non-cytotoxic concentrations were suitable for the following bio-assay experiments.
Fig. 1.
Treatments effects with water and ethanol extracts from the naturally lacto-fermented cucumber product (A, B) as well as from a commercial kimchi product (C, D) on cell viabilities of RAW 264.7 cells. Values are means ± SD (n = 8 biological determinations). Bars in the same plot not sharing a common letter (a–e) are significantly different (P < 0.05) from each other analyzed by one-way ANOVA, followed by Duncan’s multiple range test.
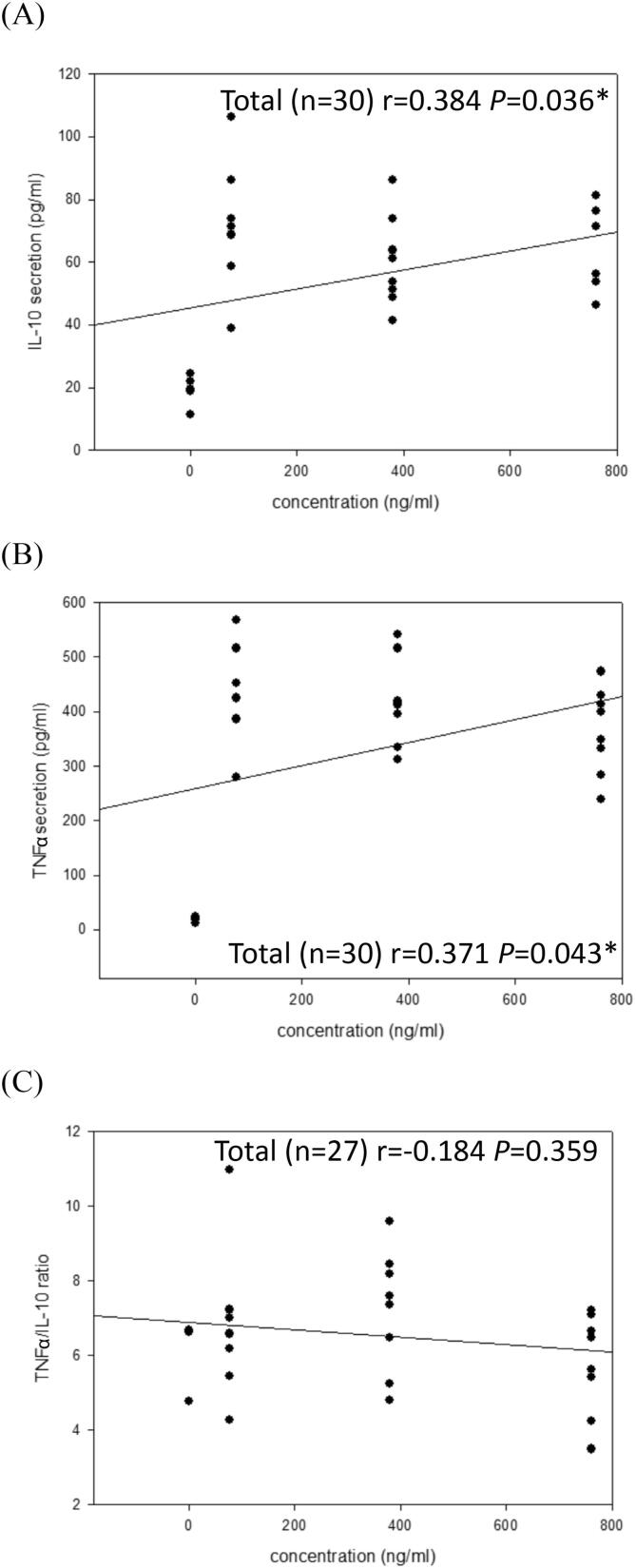
Fig. 2.
The relationships between total polyphenol contents from the CEE in the media and IL-10 secretion (A), TNF-α secretion (B) and TNF-α/IL-10 ratio (C) by LPS-stimulated RAW 264.7 macrophages under an inflammatory repair model. The associations were expressed as Pearson's product moment correlation coefficient (r). *, P < 0.05 was considered significant statistically.
Model A: Direct action of extracts from the naturally lacto-fermented cucumber product and a commercial kimchi product treated alone on RAW 264.7 cells in the absence of LPS
To assess and compare the anti-inflammatory potential of the 8-day naturally lacto-fermented cucumbers and a commercial kimchi product in vitro, RAW 264.7 cell cultures were treated with the test samples (CWE, CEE, KWE, and KEE) at the adopted non-cytotoxic concentrations for 48 h, respectively. Changes in critical pro- (IL-1β, IL-6, and TNF-α)/anti-inflammatory (IL-10) cytokines secreted by RAW 264.7 cells were determined. Table 1 shows the treatment effects with CWE, KWE, CEE and KEE on pro-/anti-inflammatory cytokine secretions by RAW 264.7 cells under a specified model A. Our results exhibited that LPS (100 ng/ml) treatment alone significantly (P < 0.05) increased both pro- and anti-inflammatory cytokine secretions, particularly TNF-α and IL-6, compared to the control, suggesting that LPS at 100 ng/ml is a suitable dose in stimulating intermediate inflammation of RAW 264.7 cells. Importantly, all test samples at the indicated concentrations dose-dependently and significantly (P < 0.05) stimulated TNF-α, but not IL-1β and IL-6, secretions by RAW 264.7 cells (Table 1) compared to that of the control, evidencing that both the 8-day naturally lacto-fermented cucumbers and a commercial kimchi product had a stimulatory activity on RAW 264.7 cells. Undoubtedly, the stimulatory activity of test samples might also cause slight but not significant inflammation in RAW 264.7 macrophages compared to that of the LPS treatment. However, commercial kimchi (KWE and KEE) demonstrated the higher stimulatory potential than the 8-day naturally lacto-fermented cucumber extracts (CWE and CEE) in stimulating macrophages, suggesting that commercial kimchi extracts (KWE and KEE) might cause the stronger inflammation than the 8-day naturally lacto-fermented cucumber extracts (CWE and CEE). Although most IL-1β, IL-6 and IL-10 levels in the treated RAW 264.7 cell cultures were too low to be detectable in this experiment, treatments with these extracts from lacto-fermented products alone might cause mild inflammation but not excess stimulation in RAW 264.7 cells.
Table 1.
Treatment effects with CWE, KWE, CEE, and KEE alone on pro- and anti-inflammatory cytokine secretions using RAW264.7 cells in the absence of LPS under a specified model A.
| Treatment | Concentration (µg/ml) |
Pro-inflammatory cytokines (pg/ml) |
Anti-inflammatory cytokine (pg/ml) | ||
|---|---|---|---|---|---|
| IL-1β | IL-6 | TNF-α | IL-10 | ||
| control | ND | ND | ND | ND | |
|
CWE |
50 | ND | ND | 302 ± 81d | ND |
| 250 | ND | ND | 320 ± 86d | ND | |
| 500 | ND | ND | 361 ± 71d | ND | |
|
KWE |
50 | ND | ND | 230 ± 68d | ND |
| 250 | ND | ND | 942 ± 234c | ND | |
| 500 | ND | ND | 1565 ± 265b | ND | |
| LPS (100 ng/ml) | 271 ± 77 | 1144 ± 136 | 3479 ± 287a | 166 ± 40 | |
| control | ND | ND | ND | ND | |
|
CEE |
10 | ND | ND | 98 ± 29d | ND |
| 50 | ND | ND | 107 ± 26d | ND | |
| 100 | ND | ND | 147 ± 32d | ND | |
|
KEE |
10 | ND | ND | 117 ± 35d | ND |
| 50 | ND | ND | 822 ± 169c | ND | |
| 100 | ND | ND | 1381 ± 176b | ND | |
| LPS (100 ng/ml) | 271 ± 77 | 1144 ± 136 | 3479 ± 287a | 166 ± 40 | |
Values are means ± SD (n = 6 biological determinations). Values within the same column in the same solvent extract sample not sharing a common superscript letter (a-d) are significantly different (P < 0.05) from each other analyzed using one-way ANOVA, followed by Duncan’s multiple range test. The ELISA kit detection limit used in this study was < 15.6 pg/ml for IL-1β, IL-6 and IL-10; <31.2 pg/ml for TNF-α. The lipopolysaccharide (LPS) alone was selected as positive control. ND, not detectable. CWE, water extract from the 8-day naturally lacto-fermented cucumbers; KWE, commercial kimchi water extract; CEE, ethanol extract from the 8-day naturally lacto-fermented cucumbers; KEE, commercial kimchi ethanol extract.
Model B: Simultaneous effects of extracts from the naturally lacto-fermented cucumber product and a commercial kimchi product on LPS-stimulated inflammation in RAW 264.7 cells
To evaluate simultaneous effects of extracts from the 8-day naturally lacto-fermented cucumber and commercial kimchi products on LPS-stimulated inflammation in vitro, RAW 264.7 cells in the presence of LPS were respectively co-treated with CWE, CEE, KWE and KEE at the adopted non-cytotoxic concentrations for 48 h. Cytokine secretion profiles by LPS-stimulated RAW 264.7 cells were measured. Table 2 shows the simultaneous effects of these extracts on pro-inflammatory and anti-inflammatory cytokine secretions by LPS-stimulated RAW 264.7 cells under a specified model B. The results showed that the treatment of LPS (100 ng/ml) alone significantly (P < 0.05) elevated pro- and anti-inflammatory cytokines production as well as IL-6/IL-10 and TNF-α/IL-10 secretion ratios compared to those of the naïve control, evidencing that LPS (an endotoxin) treatment alone indeed induced the inflammation in the treated RAW 264.7 cells under this in vitro model. Dexamethasone (DEX, a glucocorticoid) at 100 nM that was selected as a treatment control in this experiment was found to significantly (P < 0.05) inhibit IL-1β, IL-6 and TNF-α secretions, but increase IL-10 secretions by the LPS-stimulated RAW 264.7 cells compared to those of the control (Table 2), evidencing the curative effect of DEX treatment against LPS-stimulated inflammation. These observations evidenced that this in vitro cell culture model is suitable to adopt for anti-inflammatory bio-assay. Importantly, either treatments with water or ethanol extracts from these fermented products at the indicated concentrations significantly (P < 0.05) increased anti-inflammatory IL-10 secretions, particularly by CWE treatment, but inhibited IL-6/IL-10 and TNF-α/IL-10 secretion ratios by LPS-stimulated RAW 264.7 cells, particularly by CEE treatment, demonstrating their strong anti-inflammatory effects. Extracts from the 8-day naturally lacto-fermented cucumbers seem to have a better anti-inflammatory effect than those of the commercial kimchi product (Table 2). Moreover, commercial kimchi extracts (KWE and KEE) alone might cause stronger inflammation than the 8-day naturally lacto-fermented cucumber extracts (CWE and CEE) (Table 1). Therefore, just the 8-day naturally lacto-fermented cucumber extracts (CWE and CEE) were further subjected to analyze their anti-inflammatory mechanisms to unravel their inflammatory repair and/or prophylactic effects.
Table 2.
Simultaneous effects of treatments with CWE, KWE, CEE, and KEE on pro- and anti-inflammatory cytokine secretions using LPS-stimulated RAW264.7 cells under a specified model B.
| Treatment | Concentration (µg/ml) | Pro-inflammatory cytokines (pg/ml) |
Anti-inflammatory cytokine (pg/ml) |
Pro-/Anti-inflammatory cytokine ratios |
|||
|---|---|---|---|---|---|---|---|
| IL-1β (% of control) | IL-6 (% of control) | TNF-α (% of control) | IL-10 (% of control) | IL-6/IL-10 (% of control) | TNF-α/IL-10 (% of control) | ||
| Naïve control | ND | ND | ND | ND | – | – | |
|
LPS + CWE |
0 | 34 ± 11ab (100 %) | 1759 ± 581bc (100 %) | 27221 ± 7996a (100 %) | 111 ± 28e (100 %) | 15 ± 4a (100 %) | 225 ± 76a (100 %) |
| 50 | 27 ± 3cde (79 %) | 3335 ± 664a (190 %) | 25977 ± 2438ab (95 %) | 296 ± 88a (223 %) | 11 ± 2ab (73 %) | 93 ± 24c (41 %) | |
| 250 | 25 ± 2e (74 %) | 3130 ± 397a (178 %) | 28778 ± 4053a (106 %) | 283 ± 78ab (190 %) | 11 ± 4ab (73 %) | 105 ± 34bc (47 %) | |
| 500 | 31 ± 3bcde (91 %) | 2109 ± 642b (120 %) | 29017 ± 3669a (107 %) | 252 ± 61ab (147 %) | 9 ± 3bc (62 %) | 116 ± 23bc (45 %) | |
|
LPS + KWE |
0 | 34 ± 11ab (100 %) | 1759 ± 581bc (100 %) | 27221 ± 7996a (100 %) | 111 ± 28e (100 %) | 15 ± 4a (100 %) | 225 ± 76a (100 %) |
| 50 | 33 ± 4bc (97 %) | 907 ± 314d (52 %) | 21198 ± 4985bc (78 %) | 193 ± 47 cd (174 %) | 5 ± 2 cd (37 %) | 111 ± 29bc (49 %) | |
| 250 | 32 ± 2bcd (94 %) | 1303 ± 429 cd (74 %) | 18890 ± 4148c (69 %) | 160 ± 15cde(144 %) | 8 ± 3bcd (54 %) | 107 ± 16bc (47 %) | |
| 500 | 38 ± 7a (111 %) | 1740 ± 566bc (99 %) | 24598 ± 6203ab (90 %) | 181 ± 43 cd (163 %) | 9 ± 4bc (59 %) | 131 ± 26bc (58 %) | |
| LPS + DEX (100 nM) | ND | 822 ± 273d (47 %) | 9394 ± 2075e (34 %) | 213 ± 64bc (191 %) | – | – | |
|
LPS + CEE |
0 | 34 ± 11bc (100 %) | 1759 ± 581a (100 %) | 27221 ± 7996a (100 %) | 111 ± 28d (100 %) | 15 ± 4a (100 %) | 225 ± 76a (100 %) |
| 10 | 45 ± 13ab (132 %) | 937 ± 280bc (53 %) | 16860 ± 4849bcd (62 %) | 174 ± 41ab (157 %) | 6 ± 3bc (39 %) | 86 ± 19de (38 %) | |
| 50 | 36 ± 11bc (106 %) | 701 ± 201bc (40 %) | 18465 ± 4921bc (68 %) | 158 ± 40bc (142 %) | 4 ± 0c (25 %) | 113 ± 26bcd (50 %) | |
| 100 | 29 ± 4c (85 %) | 488 ± 135c (28 %) | 18570 ± 4569bc (68 %) | 146 ± 30bcd (132 %) | 4 ± 0c (25 %) | 128 ± 36bcd (57 %) | |
|
LPS + KEE |
0 | 34 ± 11bc (100 %) | 1759 ± 581a (100 %) | 27221 ± 7996a (100 %) | 111 ± 28d (100 %) | 15 ± 4a (100 %) | 225 ± 76a (100 %) |
| 10 | 46 ± 17a (135 %) | 976 ± 348b (56 %) | 17038 ± 4016bcd (63 %) | 163 ± 38bc (147 %) | 7 ± 3bc (47 %) | 106 ± 22 cd (47 %) | |
| 50 | 50 ± 14a (147 %) | 1146 ± 418b (65 %) | 20710 ± 5769b (76 %) | 141 ± 36bcd (127 %) | 9 ± 4b (60 %) | 147 ± 24bc (65 %) | |
| 100 | 40 ± 11abc (118 %) | 948 ± 330b (54 %) | 20185 ± 3046bc (74 %) | 138 ± 41bcd (124 %) | 7 ± 2bc (47 %) | 154 ± 30b (68 %) | |
| LPS + DEX (100 nM) | ND | 822 ± 273bc (47 %) | 9394 ± 2075e (34 %) | 213 ± 64a (192 %) | – | – | |
Values are means ± SD (n = 6 biological determinations). Values within the same column in the same solvent extract not sharing a common superscript letter (a-e) are significantly different (P < 0.05) from each other analyzed by one-way ANOVA, followed by Duncan’s multiple range test. The limit of detection of ELISA kits used in this study was < 15.6 pg/ml for IL-1β, IL-6 and IL-10; <31.2 pg/ml for TNF-α. The lipopolysaccharide (LPS) at 100 ng/ml alone was selected as a control; the dexamethasone (DEX) was selected as treatment control. ND, not detectable; -, not compared. CWE, water extract from the 8-day naturally lacto-fermented cucumbers; KWE, commercial kimchi water extract; CEE, ethanol extract from the 8-day naturally lacto-fermented cucumbers; KEE, commercial kimchi ethanol extract.
Model C: Inflammatory repair effect of extracts from the naturally lacto-fermented cucumber product on LPS-stimulated inflammation in RAW 264.7 cells
To unravel possible anti-inflammatory mechanisms of the 8-day naturally lacto-fermented cucumber extracts, a repair experimental model in vitro was designed for anti-inflammation bio-assay. The 8-day naturally lacto-fermented cucumber extracts (CWE and CEE) were added to cure RAW 264.7 cells for 12 h after LPS-stimulation for 12 h. Table 3 shows the CWE and CEE treatment effects on IL-6, TNF-α, and IL-10 secretions by LPS-stimulated RAW 264.7 cells under the specified repair model C. Our results evidenced that DEX treatment at 100 nM decreased significantly (P < 0.05) IL-6, TNF-α and IL-10 secretions by the LPS-stimulated RAW 264.7 cells compared to those of the control (Table 3), confirming the inhibitory and therapeutic characteristics of DEX treatment against LPS-induced inflammation. Importantly, either CWE or CEE treatments at the adopted concentrations significantly (P < 0.05) decreased IL-6, TNF-α and IL-10 secretion levels, as well as most IL-6/IL-10 and TNF-α/IL-10 secretion ratios by the LPS-stimulated RAW 264.7 cells, evidencing both CWE and CEE treatments have an anti-inflammatory effect through a repair manner. However, CWE seemed to have a better anti-inflammatory potential than CEE particularly through decreasing IL-6/IL-10 and TNF-α/IL-10 secretion ratios in this repair cell culture model C. The active components in CWE deserve to be developed as healthy foods or a therapeutic agent against inflammation in the future.
Table 3.
Treatment effects with CWE and CEE on pro- and anti-inflammatory cytokine secretions by LPS-stimulated RAW264.7 cells under a specified repair model C.
| Treatment | Concentration (µg/ml) | Pro-inflammatory cytokines (pg/ml) |
Anti-inflammatory cytokine (pg/ml) |
Pro-/Anti-inflammatory cytokine ratios |
||
|---|---|---|---|---|---|---|
| IL-6 (% of control) | TNF-α (% of control) | IL-10 (% of control) | IL-6/IL-10 (% of control) | TNF-α/IL-10 (% of control) | ||
| Naïve control | ND | 113 ± 29d (7 %) | 19 ± 4e (9 %) | – | – | |
|
LPS + CWE |
0 | 1908 ± 48a (100 %) | 1522 ± 39a (100 %) | 203 ± 43a (100 %) | 10 ± 2a (100 %) | 8 ± 2a (100 %) |
| 50 | 620 ± 185cde (33 %) | 498 ± 41b (33 %) | 95 ± 22 cd (47 %) | 7 ± 1bcd (69 %) | 6 ± 1bc (71 %) | |
| 250 | 831 ± 245bc (44 %) | 521 ± 56b (34 %) | 118 ± 26bc (58 %) | 7 ± 2bc (69 %) | 5 ± 1c (60 %) | |
| 500 | 833 ± 238bc (44 %) | 535 ± 76b (35 %) | 108 ± 24bc (53 %) | 8 ± 2b (76 %) | 5 ± 1bc (60 %) | |
|
LPS + CEE |
0 | 1908 ± 48a (100 %) | 1522 ± 39a (100 %) | 203 ± 43a (100 %) | 10 ± 2a (100 %) | 8 ± 2a (100 %) |
| 10 | 608 ± 155 cd (32 %) | 430 ± 87bc (28 %) | 68 ± 23cde (33 %) | 9 ± 2abc (88 %) | 7 ± 2abc (92 %) | |
| 50 | 534 ± 153cde (28 %) | 418 ± 79bc (28 %) | 60 ± 15e (30 %) | 10 ± 3ab (100 %) | 7 ± 2ab (92 %) | |
| 100 | 472 ± 115de (25 %) | 365 ± 79c (24 %) | 65 ± 13de (32 %) | 8 ± 2bcd (76 %) | 6 ± 1bcd (71 %) | |
| LPS + DEX (100 nM) | 420 ± 118e (13 %) | 297 ± 53c (20 %) | 76 ± 14d (37 %) | – | – | |
Values are means ± SD (n = 6 biological determinations). Values within the same column not sharing a common superscript letter (a-e) are significantly different (P < 0.05) from each other analyzed using one-way ANOVA, followed by Duncan’s multiple range test. The ELISA kit detection limit used in this study was <15.6 pg/ml for IL-1β, IL-6 and IL-10; <31.2 pg/ml for TNF-α. The lipopolysaccharide (LPS) at 100 ng/ml alone was selected as a control; the dexamethasone (DEX) was selected as treatment control. ND, not detectable; -, not compared. CWE, water extract from the 8-day naturally lacto-fermented cucumbers; CEE, ethanol extract from the 8-day naturally lacto-fermented cucumbers.
Model D: Inflammatory prophylactic effect of extracts from the naturally lacto-fermented cucumber product on LPS-stimulated inflammation in RAW 264.7 cells
An inflammatory prophylactic experimental model in vitro was designed for unravelling possible anti-inflammatory mechanisms of the 8-day naturally lacto-fermented cucumber extracts. CWE and CEE extracts were respectively added to RAW 264.7 cell cultures before LPS-stimulation. Table 4 shows the CWE and LEE treatment effects on IL-6, TNF-α, and IL-10 secretions by LPS-stimulated RAW 264.7 cells under a specified preventive model D. The results showed that DEX treatment at 100 nM decreased significantly (P < 0.05) IL-6, TNF-α and IL-10 secretions by the LPS-stimulated RAW 264.7 cells compared to those of the control (Table 4), suggesting the inhibitory and preventive effects of DEX treatment on LPS-induced inflammation. CWE and CEE treatments at the adopted concentrations inhibited most IL-6, TNF-α and IL-10 secretions, but significantly (P < 0.05) enhanced IL-6/IL-10 and TNF-α/IL-10 secretion ratios by the LPS-stimulated RAW 264.7 cells. Our results suggest that CWE and CEE treatments might have slightly preventive and inhibitory effects on pro- and anti-inflammatory cytokines secretion but did not have anti-inflammatory potential in a preventive manner.
Table 4.
Treatment effects with CWE and CEE on pro- and anti-inflammatory cytokine secretions using LPS-stimulated RAW264.7 macrophages under a specified preventive model D.
| Treatment | Concentration (µg/ml) | Pro-inflammatory cytokines (pg/ml) |
Anti-inflammatory cytokine (pg/ml) |
Pro-/Anti-inflammatory cytokine ratios |
||
|---|---|---|---|---|---|---|
| IL-6 (% of control) | TNF-α (% of control) | IL-10 (% of control) | IL-6 /IL-10 (% of control) | TNF-α/ IL-10 (% of control) | ||
| Naïve control | ND | 138 ± 29f (0.6 %) | 23 ± 6d (10 %) | – | – | |
| LPS + CWE | 0 | 1914 ± 89a (100 %) | 23983 ± 3520a (100 %) | 209 ± 59a (100 %) | 9 ± 2b (100 %) | 100 ± 33c (100 %) |
| 50 | 910 ± 249b (48 %) | 13030 ± 3846d (61 %) | 60 ± 8bc (26 %) | 15 ± 3a (175 %) | 200 ± 20c (201 %) | |
| 250 | 939 ± 195b (49 %) | 18029 ± 4693bc (85 %) | 72 ± 22b (31 %) | 14 ± 4a (161 %) | 256 ± 53bc (257 %) | |
| 500 | 759 ± 181b (40 %) | 21034 ± 6104ab (99 %) | 63 ± 13bc (27 %) | 12 ± 1a (140 %) | 329 ± 62ab (330 %) | |
| LPS + CEE | 0 | 1914 ± 89a (100 %) | 23983 ± 3520ab (100 %) | 209 ± 59a (100 %) | 9 ± 2b (100 %) | 100 ± 33c (100 %) |
| 10 | 1019 ± 90b (53 %) | 21092 ± 5814ab (99 %) | 62 ± 18b (60 %) | 16 ± 5a (192 %) | 320 ± 20ab (320 %) | |
| 50 | 997 ± 145b (52 %) | 24200 ± 6981a (114 %) | 58 ± 6b (61 %) | 16 ± 3a (186 %) | 378 ± 116a (378 %) | |
| 100 | 875 ± 116b (46 %) | 17136 ± 4731b (80 %) | 61 ± 8b (55 %) | 14 ± 2a (159 %) | 267 ± 76b (267 %) | |
| LPS + DEX (100 nM) | 185 ± 49d (18 %) | 5933 ± 1299e (35 %) | 90 ± 8c (39 %) | – | – | |
Values are means ± SD (n = 6 biological determinations). Values within the same column not sharing a common superscript letter (a-e) are significantly different (P < 0.05) from each other analyzed using one-way ANOVA, followed by Duncan’s multiple range test. The ELISA kit detection limit used in this study was < 15.6 pg/ml for IL-1β, IL-6 and IL-10; <31.2 pg/ml for TNF-α. The lipopolysaccharide (LPS) at 100 ng/ml alone was selected as a control; the dexamethasone (DEX) was selected as treatment control. ND, not detectable; -, not compared. CWE, water extract from the 8-day naturally lacto-fermented cucumbers; CEE, ethanol extract from the 8-day naturally lacto-fermented cucumbers.
Association between total active compound levels in CEE and pro-/anti-inflammatory cytokine secretion profiles using CEE-treated RAW 264.7 cells in the absence of LPS
Total active compound levels including total phenolic, flavonoid, and saponin contents in CEE from the 8-day lacto-fermented cucumbers were determined (Kao & Lin, 2023a). We found that the 8-day natural fermentation process enhanced functional phenolic, flavonoids and saponin ingredients in the lacto-fermented cucumbers (Kao & Lin, 2023a). The increased active compound contents are supposed to be produced by probiotics or released from bound compounds in the naturally lacto-fermented cucumbers through the 8-day aging process. Dietary fermented cucumbers that are abundant in diverse functional compounds may further contribute to health for their potent antioxidant functions (Wijewardhana, Gunathilaka, & Navaratne, 2019). We hypothesized that total phenolic, flavonoid, and/or saponin contents in CEE may contribute to anti-inflammatory functions. However, the effects of these total active compound levels in CEE on pro-/anti-inflammatory cytokine secretion profiles by RAW 264.7 cells remain unclear. To clarify the mystery, associations between total phenolic, flavonoid and saponin levels in CEE and pro-/anti-inflammatory cytokine secretion profiles by CEE-treated RAW 264.7 cells in the absence of LPS were analyzed. The results showed that total phenolic contents in CEE were significantly (P < 0.05) correlated with IL-10 (r = 0.384, P = 0.036*) (Fig. 2 (A)) and TNF-α (r = 0.371, P = 0.043*) (Fig. 2 (B)), but slightly (P < 0.05) correlated with the TNF-α/IL-10 secretion ratio (r = −0.184, P = 0.359) (Fig. 2 (C). Our results suggest that total phenolic content in CEE exert its anti-inflammatory effects on RAW 264.7 cells, mostly via increasing anti-inflammatory IL-10 cytokine secretions. Total flavonoid (Fig. S1) and saponin (Fig. S2) content analyses in CEE on pro-/anti-inflammatory cytokine secretion profiles showed a similar trend to total phenolic levels. As predicted, increased total phenolic, flavonoid, and/or saponin contents in CEE in the lacto-fermented cucumber product contributed to anti-inflammatory functions via regulating cytokine secretions. However, other active components in the lacto-fermented cucumber product and their corresponding mechanisms should be further explored in the future.
In our preliminary study, we found that the pH value of the developed the 8-day naturally lacto-fermented cucumbers was significantly lowered to less than 4.6 (aka high acid foods), but functional components increased while detrimental ingredients did not exceed the risk limit, suggesting the safety and health benefits (Kao et al., 2023, Kao and Lin, 2023a). In this study we further evidenced that extracts from the 8-day naturally lacto-fermented cucumbers have better anti-inflammatory effects than those of the commercial kimchi product in the experiment model B (Table 2). Either CWE or CEE treatments significantly (P < 0.05) decreased IL-6, TNF-α and IL-10 secretion levels, as well as most IL-6/IL-10 and TNF-α/IL-10 secretion ratios by the LPS-stimulated RAW 264.7 cells, suggesting that both CWE and CEE treatments have an anti-inflammatory effect through a repair manner (Table 3). CWE treatment was found to have a better anti-inflammatory potential than CEE particularly through decreasing IL-6/IL-10 and TNF-α/IL-10 secretion ratios in this repair cell culture model C (Table 3). We hypothesized that water soluble ingredients and polar molecules such as polysaccharides, gums and polyphenols in CWE may enhance macrophage immunomodulatory characteristics (Lin and Lin, 2020a, Lin and Lin, 2020b, Pan et al., 2017). Polyphenols and polysaccharides in fermented products may play a vital part and deserve to be further investigated. Unfortunately, neither CWE nor CEE treatments exerted anti-inflammatory potential in a preventive manner (Table 4). We supposed that ingested active compounds, such as high molecular weight polysaccharides and gums rich in CWE or relatively low-polar flavonoids and saponins molecules rich in CEE, might be quickly metabolized by RAW 264.7 cells, thus resulting in the lower anti-inflammatory potential in a preventive manner in vitro (Liao and Lin, 2014, Liu and Lin, 2015, Pan et al., 2017). However, increased total phenolic, flavonoid, and/or saponin contents in CEE in the lacto-fermented cucumber product still contribute to anti-inflammatory functions via regulating cytokine secretions (Fig. 2, Fig. S1, and Fig. S2) (Liao and Lin, 2020, Lin and Tang, 2007, Lin and Tang, 2008). Increased phenolics, flavonoids, different antioxidants and/or exopolysaccharides in particular probiotics-fermented products may contribute to strong anti-inflammatory effects in a repair manner (Elmansy et al., 2022). CWE, CEE and the 8-day lacto-fermented cucumber product can be developed as health foods or a therapeutic agent against inflammation in the future (Table 3), although the active compounds, such as total phenolics, flavonoids, and saponins in CWE or CEE from the lacto-fermented cucumbers might be quickly metabolized by RAW 264.7 cells (Liao and Lin, 2014, Liu and Lin, 2015).
There is a large body of evidence for supporting the vital role of plant-based fermented foods, such as soybean, cabbage and berries, in anti-inflammatory and immunomodulatory effects for promoting health and preventing disease (SaeidiFard et al., 2020, Shahbazi et al., 2021). Fermented foods have recently been suggested for preventive and therapeutic purposes on various diseases, including gastrointestinal disorder, diabetes, obesity, cardiovascular disease, cancer, neurodegenerative disorders, aging, brain and cognitive function, and Alzheimer’s disease (Baruah et al., 2022, Das et al., 2020, Kim et al., 2016, Kumar et al., 2022, Tasdemir and Sanlier, 2020). Eight-day naturally lacto-fermented cucumbers were developed for anti-inflammatory and immunomodulatory purposes (Kao et al., 2023, Kao and Lin, 2023a). In the present study, the anti-inflammatory effects of the developed 8-day naturally lacto-fermented cucumbers on RAW 264.7 macrophages were assessed. Based on pro-/anti-inflammatory cytokine secretion profile using treated RAW 264.7 macrophages under four in vitro models, both CWE and CEE from naturally lacto-fermented cucumbers were evidenced to have potential to inhibit LPS-stimulated inflammation in macrophages in a repair manner (Table 3), further suggesting the therapeutic effect of the developed 8-day naturally lacto-fermented cucumbers against inflammation (Baruah, Ray, & Halami, 2022). Importantly, CWE, CEE and the 8-day naturally lacto-fermented cucumber product may have the potential to be developed as healthy foods or a therapeutic agent against inflammation.
Even though some results were achieved in the present study, there are few limitations that should be further studied in the future. Firstly, this study is just an in vitro study, more in vivo studies should be performed to accumulate solid data for clinical and preclinical uses of the lacto-fermented cucumbers in the future. Although cytokine secretion assay could predict inflammation status but it is not the end-point assay, other inflammation indicators, such as nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2) and prostaglandin E2 (PGE2) production amounts by the treated cells also could be selected as another suitable assay to state the inflammatory response. Even if total phenolic, flavonoid and saponin contents which are considered as general functional ingredients in the developed naturally lacto-fermented cucumbers have been analyzed (Kao & Lin, 2003a), more detail data concerning individual potential active ingredients in the extracts and lacto-fermented cucumbers should be identified using high-performance liquid chromatography (HPLC), gas chromatography (GC), or ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) in the future. In addition, active compounds could be both the metabolite from the cucumber and also from health probiotics. Therefore, most fermented vegetables have complicated chemistries, making it difficult to simply attribute its original health benefits to prebiotics, probiotics, synbiotics and/or postbiotics produced during the fermentation process (Castellone et al., 2021, Garrote et al., 2015, Zieliński et al., 2017). Moreover, the anti-oxidative effect and western blot about particular anti-inflammatory cell signaling pathways should be evaluated in the future. More research data should be achieved to clarify functional characteristics of the lacto-fermented cucumbers. In this study, Duncan’s range test was employed for post-hoc analyses which a statistical method may induce type I error rate, although the type I error rate may be reduced by moderately minimizing the significance level, leading to this test is not recommended by some statisticians. Recently, Tukey-Kramer test is suggested for analyzing to avoid possible type I error rate. Resolving these limitations to accumulate more useful research data may contribute to a comprehensive understanding of the functional characteristics of lacto-fermented cucumbers. This study is the first to analyze anti-inflammatory characteristics of a naturally developed lacto-fermented cucumber product using simple RAW 264.7 cell culture models, although the experiment in this manuscript is still a preliminary pharmacodynamic evaluation and more pharmacodynamic evaluation and potential mechanism should be explored in the future.
Conclusions
In the present study the results evidenced that both water and ethanol extracts from the 8-day naturally lacto-fermented cucumber product had a mild stimulation to macrophages but strong potential to inhibit LPS-induced inflammation in macrophages in a repair manner. The CWE exerted a better effect than CEE. Moreover, the total phenolic, flavonoid, and saponin contents in CEE were significantly (P < 0.05) correlated with IL-10 (r = 0.384, P = 0.036*) and TNF-α (r = 0.371, P = 0.043*) secretions, but slightly correlated with the TNF-α/IL-10 secretion ratio (r = −0.184, P = 0.359), respectively. Our results suggest that increased total phenolic, flavonoid, and saponin contents in CEE contributed to anti-inflammatory functions via regulating cytokine secretions. CWE, CEE and the 8-day naturally lacto-fermented cucumber product may have the potential to be developed as healthy foods or a therapeutic agent against inflammation. Our study is the first to evidence anti-inflammatory effects of a naturally developed lacto-fermented cucumber product using RAW 264.7 macrophages in vitro.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was kindly supported by research grants 110AS-14.1.1-ST-a4 from the Council of Agriculture, and NSTC 112-2320-B-005-013 from the National Science and Technology Council, Executive Yuan, Taipei, Taiwan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101039.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agatemor U.M.M., Nwodo O.F.C., Anosike C.A. Anti-inflammatory activity of Cucumis sativus L. British Journal of Pharmaceutical Research. 2015;8:1–8. [Google Scholar]
- Baruah R., Ray M., Halami P. Preventive and therapeutic aspects of fermented foods. Journal of Applied Microbiology. 2022;132:3476–3489. doi: 10.1111/jam.15444. [DOI] [PubMed] [Google Scholar]
- Bhatia R., Singh S., Maurya R., Bhadada S.K., Bishnoi M., Chopra K.…Kondepudi K.K. In vitro characterization of lactic acid bacterial strains isolated from fermented foods with anti-inflammatory and dipeptidyl peptidase-Ⅳ inhibition potential. Brazilian Journal of Microbiology. 2023;54:293–309. doi: 10.1007/s42770-022-00872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S.S., Sheikha A.F.E., Hammami R., Kumar A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? Journal of Functional Foods. 2020;70 [Google Scholar]
- Castellone V., Bancalari E., Rubert J., Gatti M., Neviani E., Bottari B. Eating fermented: Health benefits of LAB-fermented foods. Foods. 2021;10:2639. doi: 10.3390/foods10112639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia R., Phukon L.C., Abedin M.M., Padhi S., Singh S.P., Rai A.K. Bioactive peptides in fermented foods and their application: Acritical review. Systems Microbiology and Biomanufacturing. 2023;3:88–109. [Google Scholar]
- Dala-Paula B.M., Starling M.D.F.V., Gloria M.B.A. Vegetables consumed in Brazilian cuisine as sources of bioactive amines. Food Bioscience. 2021;40 [Google Scholar]
- Das G., Paramithiotis S., Sivamaruthi B.S., Wijaya C.H., Suharta S., Sanlier N.…Patra J.K. Traditional fermented foods with anti-aging effect: A concentric review. Food Research International. 2020;134 doi: 10.1016/j.foodres.2020.109269. [DOI] [PubMed] [Google Scholar]
- Diez-Ozaeta I., Astiazaran O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111133. [DOI] [PubMed] [Google Scholar]
- Elmansy E.A., Elkady E.M., Asker M.S., Abdou A.M., Abdallah N.A., Amer S.K. Exopolysaccharide produced by Lactiplantibacillus plantarum RO30 isolated from Romi cheese: Characterization, antioxidant and burn healing activity. World Journal of Microbiology and Biotechnology. 2022;38:245. doi: 10.1007/s11274-022-03439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrote G.L., Abraham A.G., Rumbo M. Is lactate an undervalued functional component of fermented food products? Frontiers in Microbiology. 2015;6:629. doi: 10.3389/fmicb.2015.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C.C., Lin J.Y. Culture condition optimization of naturally lacto-fermented cucumbers based on changes in detrimental and functional ingredients. Food Chemistry: X. 2023;19 doi: 10.1016/j.fochx.2023.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C.C., Lin J.Y. Lactiplantibacillus plantarum subsp. plantarum BCRC10069 co-fermented cucumber safety and functional characteristics. Food Bioscience. 2023;55 doi: 10.1016/j.fbio.2023.103085. [DOI] [Google Scholar]
- Kao C.C., Wang H.M., Tsai S.J., Lin J.Y. Sensory and microbial analyses on naturally lacto-fermented cucumbers. International Journal of Gastronomy and Food Science. 2023;32 [Google Scholar]
- Kim B., Hong V.M., Yang J., Hyun H., Im J.J., Hwang J.…Kim J.E. A review of fermented foods with beneficial effects on brain and cognitive function. Preventive Nutrition and Food Science. 2016;21:297–309. doi: 10.3746/pnf.2016.21.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.R., Azizi N.F., Yeap S.K., Abdullah J.O., Khalid M., Omar A.R.…Alitheen N.B. Clinical and preclinical studies of fermented foods and their effects on Alzheimer’s disease. Antioxidants. 2022;11:883. doi: 10.3390/antiox11050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Dong L., Liu Y., Chen Q., Wu Z., Liu L.…Liu L. Ultrasound and enzyme assisted preparation of novel lactoferrin-cereal phenolic acid conjugates: Structural, physiochemical and functional properties. Food Chemistry. 2024;435 doi: 10.1016/j.foodchem.2023.137572. [DOI] [PubMed] [Google Scholar]
- Liao Y.R., Lin J.Y. Quercetin, but not its metabolite quercetin-3-glucuronide, exerts prophylactic immuno-stimulatory activity and therapeutic anti-inflammatory effect on lipopolysaccharide-treated mouse peritoneal macrophages ex vivo. Journal of Agricultural and Food Chemistry. 2014;62:2872–2880. doi: 10.1021/jf405630h. [DOI] [PubMed] [Google Scholar]
- Liao Y.R., Lin J.Y. Quercetin modulates cytokine expression and inhibits TLR2 expression and STAT3 activation in mouse activated inflammatory macrophages. Journal of Exploratory Research in Pharmacology. 2020;5:31–41. [Google Scholar]
- Lin H.C., Lin J.Y. Characterization of guava (Psidium guajava Linn) seed polysaccharides with an immunomodulatory activity. International Journal of Biological Macromolecules. 2020;154:511–520. doi: 10.1016/j.ijbiomac.2020.03.137. [DOI] [PubMed] [Google Scholar]
- Lin H.C., Lin J.Y. M1 polarization but anti-LPS-induced inflammation and anti-MCF-7 breast cancer cell growth effects of five selected polysaccharides. Evidence-based Complementary and Alternative Medicine. 2020;2020:9450246. doi: 10.1155/2020/9450246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.C., Lin J.Y. Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. Journal of Agricultural and Food Chemistry. 2011;59:184–192. doi: 10.1021/jf103581r. [DOI] [PubMed] [Google Scholar]
- Lin J.Y., Tang C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chemistry. 2007;101:140–147. [Google Scholar]
- Lin J.Y., Tang C.Y. Total phenolic contents in selected fruit and vegetable juices exhibit a positive correlation with interferon-γ, interleukin-5, and interleukin-2 secretions using primary mouse splenocytes. Journal of Food Composition and Analysis. 2008;21:45–53. [Google Scholar]
- Liu C.J., Lin J.Y. Quercetin uptake and metabolism by murine peritoneal macrophages in vitro. Journal of Food and Drug Analysis. 2015;23:692–700. doi: 10.1016/j.jfda.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang J., Deng Z. Editorial: Changes in food functional components during innovative processing technologies and delivery systems, digestion, and metabolism. Frontiers in Nutrition. 2023;10:1200010. doi: 10.3389/fnut.2023.1200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Wu J., Ho C., Badmaev V. Effects of water extract of Curcuma longa (L.) roots on immunity and telomerase function. Journal of Complementary and Integrative Medicine. 2017;14:20150107. doi: 10.1515/jcim-2015-0107. [DOI] [PubMed] [Google Scholar]
- Paul A.K., Lim C.L., Apu M.A.I., Dolma K.G., Gupta M., de Lourdes Pereira M.…Nissapatorn V. Are fermented foods effective against inflammatory diseases? International Journal of Environmental Research and Public Health. 2023;20:2481. doi: 10.3390/ijerph20032481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SaeidiFard N., Djafarian K., Shab-Bidar S. Fermented foods and inflammation: A systemic review and meta-analysis of randomized controlled teials. Clinical Nutrition ESPEN. 2020;35:30–39. doi: 10.1016/j.clnesp.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Sanlier N., Gokcen B.B., Sezgin A.C. Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition. 2019;59:506–527. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- Shahbazi R., Sharifzad F., Bagheri R., Alsadi N., Yasavoli-Sharahi H., Matar C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients. 2021;13:1516. doi: 10.3390/nu13051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Sharma L., Sandhu K.S. In: Antioxidants in vegetables and nuts-properties and health benefits. Nayik G.A., Gull A., editors. Springer; Singapore: 2020. Cucumber (Cucumis sativus L.) pp. 333–340. [DOI] [Google Scholar]
- Skowron K., Budzynska A., Grudlewska-Buda K., Wiktorczyk-Kapischke N., Andrzejewska M., Walecka-Zacharska E., Gospodarek-Komkowska E. Two faces of fermented foods-The benefits and threats of its consumption. Frontiers in Microbiology. 2022;13 doi: 10.3389/fmicb.2022.845166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir S.S., Sanlier N. An insight into the anticancer effects of fermented foods: A review. Journal of Functional Foods. 2020;75 [Google Scholar]
- Wijewardhana U.S., Gunathilka U.G.S.A., Navaratne S.B. Determination of total phenolic content, radical scavenging activity and total antioxidant capacity of Cinnamon bark, Black cumin seeds and garlic. International Research Journal of Advanced Engineering and Science. 2019;4:55–57. [Google Scholar]
- Wuyts S., Beeck W.V., Allonsius C.N., van den Broek M.F.L., Lebeer S. Applications of plant-based fermented foods and their microbes. Current Opinion in Biotechnology. 2020;61:45–52. doi: 10.1016/j.copbio.2019.09.023. [DOI] [PubMed] [Google Scholar]
- Yeh T.H., Lin J.Y. Acorus gramineusand and Euodia ruticarpa steam distilled essential oils exert anti-inflammatory effects through decreasing Th1/Th2 and pro-/anti-inflammatory cytokine secretion ratios in vitro. Biomolecules. 2020;10:338. doi: 10.3390/biom10020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li Y., Ren X., Zhang X., Wu Z., Liu L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134231. [DOI] [PubMed] [Google Scholar]
- Zhao N., Lai H., He W., Wang Y., Huang Y., Shi Q.…Ge L. Assessment of biogenic amine and nitrite production in low-salt Paocai during fermentation as affected by reused brine and fresh brine. Food Bioscience. 2021;41 [Google Scholar]
- Zieliński H., Surma M., Zielińska D. In: Fermented Foods in Health and Disease Prevention. Frías J., Martínez-Villaluenga C., Peñas E., editors. Elsevier; 2017. The naturally fermented sour pickled cucumbers; pp. 503–516. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.