Abstract
Due to huge diversity and dynamic competition, the human gut microbiome produces a diverse array of antimicrobial peptides (AMPs) that play an important role in human health. The gut microbiome has an important role in maintaining gut homeostasis by the AMPs and by interacting with other human organs via established connections such as the gut–lung, and gut–brain axis. Additionally, gut AMPs play a synergistic role with other gut microbiota and antimicrobials to maintain gut homeostasis by fighting against multi-antibiotic resistance (MAR) bacteria. Further, conventional antibiotics intake creates a synergistic evolutionary pressure for gut AMPs, where antibiotics and gut AMPs fight synergistically against MAR. Overall, gut AMPs are evolving under a complex and highly synergistic co-evolutionary pressure created by the various interactions between gut microbiota, gut AMPs, and antibiotics; however, the complete mechanism is not well understood. The current review explores the synergistic action of gut AMPs and antibiotics along with possibilities to fight against MAR bacteria.
Keywords: gut microbiota, gut peptides, multi-antibiotic resistance, co-evolution
1. Introduction
The rapid emergence of MAR and bacterial infections are global health concerns that urgently need to be addressed. The unavailability of new antibiotics and failure of available therapeutic strategies due to resistance development results in severe health complications and a sharp rise in deaths throughout the world [1,2]. In light of these facts, there is an urgent need for new antimicrobials and the development of new antimicrobial therapeutic strategies with effective outcomes to win the battle against MAR. AMPs are one of the promising options to fight against MAR due to their ubiquitous availability and diverse activity spectrum [3,4]. Additionally, the amenability of AMPs to bioengineering and drug repurposing may also play an important role in the development of new strategies to treat MAR [5,6,7]. Interestingly, the human gut is a complex environment where the cohabitation of pathogens with a beneficial gut microbiome and host appeases the synergistic co-evolution and action of gut AMPs and antibiotics. AMPs are also known to have multiple antimicrobial properties within a single peptide including membrane permeabilization and inhibition of both transcription and translation [8]. In the complex environment of the gut, high antimicrobial strength and complexity are observed in the tightly synchronized secretion of AMPs enriched with interdependent properties [9]. Host defense peptide-producing cells in the gut also take advantage of this synergistic action of gut AMPs in specific combinations that result in higher efficiency against pathogens at low concentrations. Similarly, the synergistic action of gut AMPs is observed with conventional antibiotics and could be used to develop new therapeutics against MAR. Interestingly, because of their known benefits, AMP-based drugs are now under consideration by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) [10,11]. Although gut AMPs have already been discussed extensively as a potential alternative to fighting against MAR, new strategies are required to control the development and evolution of rapid resistance [12,13,14]. Also, it is important to understand the synergistic and rapid evolutionary process of gut AMPs with antibiotics. Here in the present review, we discuss the use of gut microbiota-produced AMPs in conjugation with conventional antibiotics, their synergistic co-evolution, and their action in controlling MAR.
2. Influence of Antibiotics on Gut Microbiota, Susceptibility to Infections, and Resistance Development
The most frequent and significant factor altering the normal gut microbiome composition and function is the use of antibiotics; however, many other factors that might impair the beneficial gut microbiota include mental and physical stress, radiation treatment, altered gut peristalsis, gastrointestinal infections, and dietary changes [15]. Antibiotics have a major impact on changing the gut microbiota, resulting in decreased bacterial diversity and increased numbers of some taxa [16]. This change in gut microbiome further results in the altered production of AMPs produced by gut microbiota and their associated functions impacting host immunity. Additionally, antibiotics’ activity spectrum, mode of action, potency, pharmacokinetics, dosage, and length of administration are also major factors that influence the gut AMPs and microbiome [17]; however, the presence of preexisting antimicrobial resistance genes in an individual’s microbiome is another concerning factor. Changes in the variety of gut bacteria can result in Clostridium difficile infection, which is naturally resistant to many antibiotics [18]. Other unintended consequences of antibiotic use on gut microbiota include selection for a reservoir of bacterial antibiotic resistance genes, and progression of horizontal gene transfer between bacterial strains that affects the expression, production, and regulation of gut AMPs further leading to immune dysregulation and antibiotic resistance development [16].
Antibiotics affect the local gut immune system by changing the composition of the gut resident microbiota and their metabolites, specifically AMPs. It has been shown that post-antibiotic treatment, the small intestine showed lower IL-17 and INF-γ production, while the colon showed decreased numbers of Treg cells. This suggests that antibiotics induce altered host–microbiota interactions that cause immune imbalance [19]. Additionally, the gut microbiota stimulates mucin production, whereas antibiotics cause the weakening of the mucus barrier, making the body more vulnerable to bacterial invasion and subsequent infections [20]. Intestinal infections may be brought on by newly acquired pathogens or by the overgrowth and pathogenic potential of opportunistic microorganisms due to changes in the bacterial populations that ordinarily inhabit the gut lumen. Numerous studies on infants receiving antibiotics, particularly preterm ones, have been conducted. The normal bacterial microbiota of infants is changed by treatment with different antibiotics, such as cephalexin, gentamicin, vancomycin, and erythromycin, by increasing the percentage of potentially pathogenic Enterobacteriaceae and decreasing the number of bacteria like Bifidobacteriaceae, Bacilli, and Lactobacillus which are part of the healthy microbiota [21]. Overall, antibiotics displayed a significant role in the modulation of gut microbiota that leads to infection susceptibility at one end and resistance development as another counterpart.
3. Interplay of Gut Microbiota with Gut AMPs
Gut microbiota plays an essential role in the regulation of the host defense system by maintaining gut homeostasis. Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria form the majority of the gut microbiome [22]. The diverse array of gut AMPs produced by gut microbiota plays an important role in various functional activities in the gut such as immunomodulatory activities and protection against pathogens by disrupting bacterial cell membranes and halting the RNA and DNA synthesis of metabolism [23]. Bacteriocins are the major bacterially produced gut AMPs and efficiently compete with other microbes in the gut. However, much of the gut microbiome’s diversity is still unknown; a study of some isolated microbes and metagenomic analysis suggested that there are many unrevealed classes of antibiotics and AMP-producing microbes present in the gut that are as yet unknown [24]. Gut microbiota-derived AMPs have been reported to protect against various disease-causing pathogens in the human gut (Table 1). A bacteriocin, Abp118, produced by a gut microbe Lactobacillus salivarius UCC118 in the gut is confirmed to protect against the foodborne pathogen Listeria monocytogenes. It has been confirmed that mutant L. salivarius UCC118, expressing the cognate Abp118 immunity protein AbpIM, failed to protect against L. monocytogenes infections in mice [25]. Another bacteriocin, thuricin CD, produced by Bacillus thuringiensis DPC 6431 has been shown to have efficient killing potential against disease-causing clinical isolates of C. difficile without any antagonistic effect on commensal gut microbiota [26]. Bacteriocin encoded by pheromone-responsive plasmids is common in enterococcus strains residing in the gut which are reported as gut commensals as well as for casing hospital-acquired infections [27]. Bacteriocin 21, produced by conjugative plasmid pPD1 of Enterococcus faecalis, is demonstrated to protect against vancomycin-resistant enterococci without affecting the other commensal microbiota in the gut. Interestingly, E. faecalis containing pPD1 plasmid outcompetes and replaced other E. faecalis lacking pPD1. This suggests that gut bacteriocin can also regulate the niche in the gut and can be used as potential therapeutic peptides able to target MAR bacteria specifically [28]. Another study showed that microcins produced by a probiotic bacterium Escherichia coli Nissle 1917 (EcN) can regulate inter- and intra-species competition among the Enterobacteriaceae and other related pathogens in the inflamed gut environment and are suggested as potential narrow-spectrum therapeutic agents against enteric pathogens [29].
Table 1.
Gut microbiota-produced AMPs and involvement in the treatment of different diseases.
| Gut AMPs | Producing Bacteria | Targeted Pathogens or Diseases | References |
|---|---|---|---|
| Bacteriocin Abp118 | L. salivarius | Listeriosis | [30] |
| Bacteriocin OR-7 | L. salivarius NRRLB | Campylobacter jejuni | [31] |
| Bactofencin A | L. salivarius | Antilisterial, antistaphylococcal | [32] |
| Lactocin AL705 | L. curvatus | Listeriosis | [33] |
| Lactocin 160 | L. rhamnosus |
Escherichia coli
Bordetella pertussis |
[34] |
| Lacticin3147 | Lactococcus lactis DPC3147 | C. difficile-associated diarrhea (CDAD) | [35] |
| Garvicin ML | L. garvieae | Streptococcus pneumonia | [36] |
| Nisin Z | L. lactis | Immunomodulatory effect | [37] |
| Nisin F | L. lactis | Respiratory infection | [38] |
| Nisin | L. lactis | Meningitis, sepsis, pneumonia | [39] |
| Nisin Z | L. lactis | Enteric pathogens | [37] |
| Nisin A | L. lactis | Colorectal cancer | [40] |
| Pediocin PA1 | Pediococcus acidilactici | Listeriosis | [41] |
| Pediocin AcH | P. acidilactici | Enteric pathogens | [37] |
| Enterocin CRL35 | Enterococcus mundtii RL35 | Listeriosis | [42] |
| Avicin | E. avium | Listeriosis | [43] |
| Enterocin P | E. faecium P13 | Enteric pathogens | [44] |
| Piscicolin 126, carnobacteriocin | Carnobacterium maltaromaticum | Listeriosis | [45] |
| Kimchichin | Leuconostoc citreum GJ7 | Salmonella typhi | [46] |
| Erwinaocin NA4 | Erwinia carotovora NA4 | Coliphage | [47] |
Gut-epithelium-derived peptides are also reported to have potential antimicrobial activities against gut pathogens. In the gastrointestinal tract, enterocytes and Paneth cells are the primary cells responsible for the production of AMPs; however, macrophages, dendritic cells, neutrophils, and lymphocytes present in the lamina propria can also produce AMPs [48,49]. Defensins are the major AMPs secreted within the intestinal mucosa. The α and β defensins are abundant AMPs in the gut which are primarily secreted by Paneth and epithelial cells, respectively, in the intestine and the colon [50]. Further, it has been reported that the secretion of gut AMPs by Paneth cells is regulated and stimulated by exposure to live pathogens (both Gram-positive and Gram-negative) or bacterial products such as lipopolysaccharide, lipoteichoic acid, lipid A, and muramyl dipeptide [51]. In another study, the gut resident Lactobacillus population exhibited a correlation with the gene expression of α defensins, where α defensin gene expression is restored in antibiotic-treated mice by Lactobacillus administration. Further, it has been confirmed that α defensin gene expression by Paneth cells is regulated by commensal bacteria via the TLR-MyD88 signaling pathway that provides a deeper understanding of the involvement of gut microbiota and AMPs in gut homeostasis [52]. Overexpression of α defensin 5 is found associated with a severe reduction in the colonization of segmented filamentous bacteria that are further linked with reduced levels of Th17 cells in the lamina propia and suggests the role of α defensins in the regulation of commensal microbiota [53]. Gut epithelial-produced β defensins 2 and 3 were reported to reduce the intestinal damage caused by a gut pathogen Salmonella typhimurium via enhancing the probiotic activity of Enterococcus faecium by alteration of cytokine expression [54]. Similarly to defensins, cathelicidins are also reported to produce and act against gut pathogens by improving the gut epithelial barrier. In a recent, cathelicidin-WA has been shown to improve host defense and epithelial barrier functions by reducing enterohemorrhagic Escherichia coli-induced inflammation and microbiota reduction in the intestine of mice [55]. Cathelicidin-related antimicrobial peptides (CRAMP) were found to protect against an enteric pathogen, Citrobacter rodentium, by reducing epithelial cell damage and systemic clearance of infection [56]. In another study, cathelicidins significantly improved the gut barrier against pathogens in mouse colon mucosa where endogenous stimulation or administration of cathelicidin is able to clear the infection caused by Escherichia coli O157:H7 and also regulate the gut microbiota balance; this aids mucosal homeostasis [57,58]. Other major gut peptides are regenerating AMPs (RegAMP) which are soluble lectins and mainly produced by Paneth cells. A RegAMP, RegIIIγ, is demonstrated to play an important role in maintaining gut homeostasis by spatial segregation of gut microbiota and host in the intestine [59]. Another study reported that RegIIIγ can protect against L. monocytogenes infection via MyD88-mediated conditioning of gut epithelium [60]. Further, RegAMP is reported to have a role in pathogen clearance that is dependent on the presence of initial healthy gut microbiota and it has been suggested that gut microbiota and gut AMPs are the key factors that regulate the host response during antibiotic treatment [61]. Overall, available reports suggest a complex relationship between gut-epithelium-derived AMPs and gut microbiota; however, further studies are required to explore the regulatory switches that drive the production of gut epithelium AMPs in response to specific gut commensals or pathogens.
4. Synergistic Action of Gut AMPs with Conventional Antibiotics
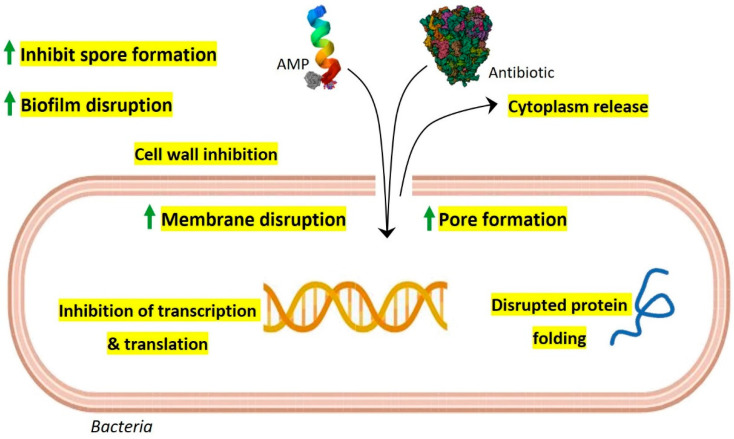
Due to the rapid emergence of multidrug resistance and the reduced efficacy of conventional antibiotics, the synergistic action of gut AMPs with antibiotics is explored and suggested as a new approach to control drug-resistant bacteria (Table 2). Interestingly, AMPs display multiple mechanisms of action at a time that include membrane pore formation, inhibition of cell wall synthesis, biofilm disruption, inhibition of spore formation, and inhibition of protein synthesis and folding, along with inhibition of DNA and RNA synthesis [12]. Especially in the complex gut environment with the possibility of numerous unknown interactions, the multiple-mode-of-action scenario of AMPs is intriguing. The synergistic action of conventional antibiotics with gut AMPs is possibly benefited by extended pore opening on the target cell membrane, increased membrane permeabilization, and subsequently increased repair time that further results in altered bacterial intracellular functions and overall bactericidal activity (Figure 1). In one of our previous studies, we have shown that laterosporulin10, a defensin-like bacteriocin produced by Brevibacillus laterosporus SKDU10, exhibits a synergetic effect with rifampicin against Mycobacterium tuberculosis H37Rv. It is confirmed that the addition of 0.25 µM laterosporulin10 results in a four-fold reduction of the rifampicin MIC values against M. tuberculosis [62]. Gut AMPs, nisin Z, and pediocin PA-1 including colistin were reported to have potential synergistic effects against MAR P. fluorescens when used in combination with antibiotics such as kanamycin, tetracycline, and chloramphenicol [63]. Nisin is also reported to have synergistic antimicrobial action with peptidoglycan-modulating antibiotics and ramoplanin, and exhibits promising activity against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci (VRE). Furthermore, nisin demonstrates improved antibiofilm and antibacterial activity against E. faecalis by exhibiting synergistic effects with antibiotics such as penicillin, ciprofloxacin, and chloramphenicol [64]. A different study showed the synergistic effects of nisin with several antibiotics, including penicillin, amoxicillin, tetracycline, streptomycin, and ceftiofur against the swine pathogen Streptococcus suis that is known to cause severe infections in pigs [65]. Another study reported the synergic effects of subtilosin with clindamycin and metronidazole when used against Gardnerella vaginalis, which causes bacterial vaginosis [66]. In vitro, the activity of various human AMPs, LL-37, HBD1 to HBD3, HNP1, and HD5 have been checked against C. difficile in combinations of different antibiotics including tigecycline, moxifloxacin, piperacillin-tazobactam, and meropenem. Interestingly, LL-37 and HBD3 were found to have synergistic action against C. difficile with all the tested antibiotics [67]. Cryptdin 2, an AMP produced by Paneth cells, showed a synergistic effect against MAR S. typhimurium when used in combination with ampicillin [68]. HNP-1 was also confirmed to exhibit synergistic action with rifampicin and isoniazid against M. tuberculosis H37Rv [69]. Further, LL-37-derived membrane active analogs, FK13-a1 and FK13-a7, showed synergistic action against multidrug-resistant P. aeruginosa (MDRPA) and methicillin-resistant S. aureus (MRSA) when used in combination with chloramphenicol [70]. Next, LL-37 and colistin are reported to have synergistic action against MAR carbapenem-resistant P. aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii when used in combination with the antibiotic azithromycin [71]. In a different study, short-cationic AMPs exhibited a synergistic effect with the antibiotics polymyxin B, erythromycin, and tetracycline against MDRPA [72]. A pilot study confirmed the synergistic action of colistin and the antibiotic tobramycin against P. aeruginosa [73]. Although multiple reports are available with improved results concerning the synergistic actions of gut AMPs with conventional antibiotics, the specific mechanisms of action need to be studied in further detail. However, in light of the evidence and analyzed results, it is possible to develop synergistic combinations of gut AMPs and antibiotics for the treatment of MAR human pathogens.
Table 2.
Gut AMPs in synergy with conventional antibiotics.
| Gut AMPs | Antibiotics | Target | References |
|---|---|---|---|
| Nisin | Ramoplanin | MRSA | [74] |
| Polymyxin E Clarithromycin | P. aeruginosa | [75] | |
| Amoxicillin Penicillin Streptomycin Ceftiofur Tetracycline |
S. suis | [65] | |
| Nisin Z | Ampicillin Chloramphenicol Kanamycin Lincomycin Penicillin G Rifampicin Streptomycin Tetracycline Vancomycin |
P. fluorescens LRC-R73 and its Penicillin-resistant/Streptomycin-resistant/Lincomycin-resistant/Rifampicin-resistant variant | [63] |
| Lacticin 3147 | Polymyxin B | S. aureus 5247 | [76] |
| Actagardine | Ramoplanin Metronidazole Vancomycin |
C. difficile | [77] |
| Thuricin CD | Ramoplanin | C. difficile | [77] |
| Vancomycin | C. difficile | [77] | |
| Subtilosin A | Clindamycin phosphate Metronidazole |
G. vaginalis | [66] |
| Lauramide arginate Ester poly-lysine |
G. vaginalis | [66] | |
| PsVP-10 | Chlorhexidine |
S. mutans
S. sobrinus |
[78] |
| Plantaricin E, F, J, K | Several antibiotics | C. albicans | [79] |
| Colistin | Tobramycin | P. aeruginosa | [73] |
| Cryptdin 2 | Ampicillin | S. typhimurium | [68] |
| Laterosporulin10 | Rifampicin | M. tuberculosis H37Rv | [62] |
| Colistin | Azithromycin |
A. baumannii
K. pneumoniae P. aeruginosa |
[71] |
| LL-37 | Azithromycin |
A. baumannii
K. pneumoniae P. aeruginosa |
[71] |
| Human defensin 5 (HD5) |
Meropenem | C. difficile | [67] |
| Human neutrophil peptide-1 (HNP1) | Rifampicin | M. tuberculosis H37Rv | [69] |
| Human β-defensin 3 (HBD3) |
Meropenem Moxifloxacin Piperacillin-Tazobactam Tigecycline |
C. difficile | [67] |
Figure 1.
Possible model for synergistic antimicrobial activity of gut AMPs with conventional antibiotics. As per their membrane-acting properties, continuous pore formation and increased membrane permeabilization by AMPs allow more influx of antibiotics and AMPs which results in efficient bactericidal activity along with improved targeting of intracellular components such as transcription, protein synthesis machinery, and protein folding. Gut AMPs might also facilitate enhanced biofilm disruption and inhibition of spore formation when used in combination with antibiotics.
5. Gut AMPs, Conventional Antibiotics, and Evolution of Resistance Development
A major global public health concern is bacterial resistance to small-molecule antibiotics that are already on the market. The global spread of antibiotic-resistant bacteria has created the possibility of a post-antibiotic age in which ordinary illnesses and small wounds could develop into potentially fatal conditions. Such resistance has resulted in the creation of multidrug-resistant bacteria over the past few decades, which can both endanger healthy people and cause serious infections in immunocompromised patients. For instance, hospital-acquired infections with ampicillin-resistant E. coli, vancomycin-resistant E. faecalis, and methicillin-resistant Staphylococcus aureus (MRSA) have all increased in frequency [80,81]. Thus, there is an urgent need for novel antimicrobial strategies given the rising threat of MAR bacteria.
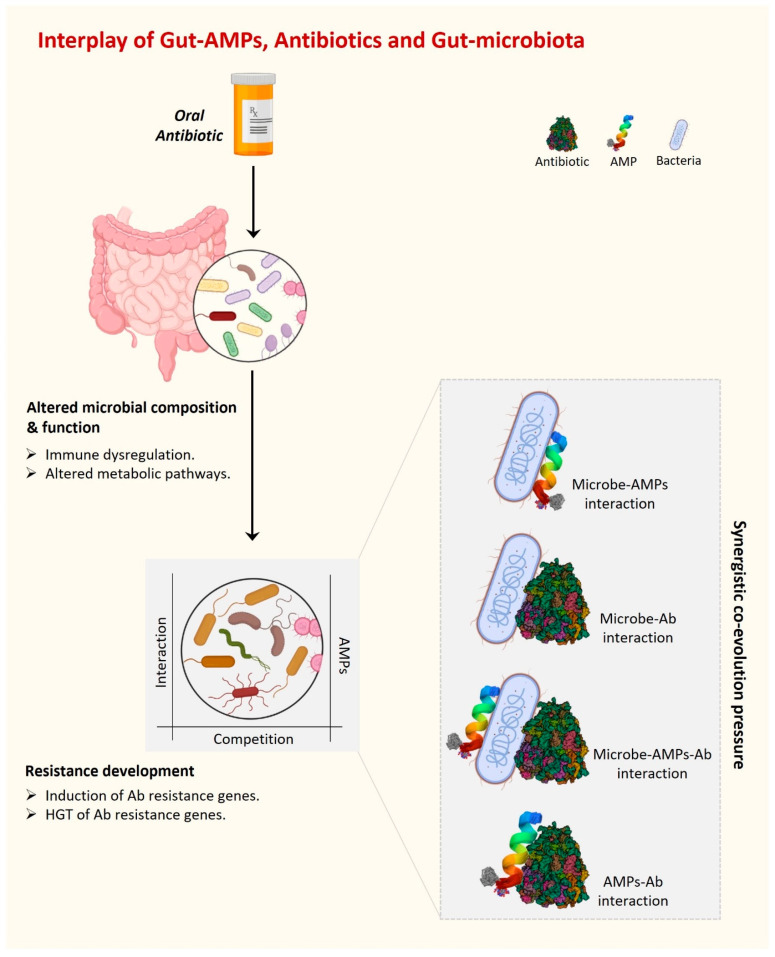
Antibiotic misuse has contributed to the emergence of MAR organisms. MAR infections are a leading source of morbidity and mortality worldwide [57]. Microbes can create and use defense and resistance mechanisms against the substances used to eradicate them in a complex environment such as the human gut, which is the home of over 100 trillion bacteria. Interestingly, not only the external antibiotics but also the antimicrobial substances produced by competitors present a challenge for the gut resident bacterial community to survive. Further, in the case of dysbiosis, an additional competition force exists between beneficial and harmful gut microbiota. AMPs are one such strategy used by bacteria (beneficial or harmful) to kill their competitors present in the surrounding complex environment. In addition to all of this, host-gut-derived AMPs are also present in the gut under the regulatory pressure of foreign AMPs, antibiotics, and the presence of their producers. Overall, there are multiple dynamic interactions present in the complex environment of the gut between various gut AMPs and antibiotics, whether internal or external. Together, these dynamic interactions and regulatory pressures create an evolutionary force under which microorganisms acquire a fair chance to evolve survival strategies and eventually develop antibiotic resistance (Figure 2). A pool of resistant genes belonging to several classes of antibiotics has been identified in a recent metagenomic study of gut resistome conducted across different continents [82].
Figure 2.
The interplay of gut AMPs, antibiotics, and gut microbiota is driven by various interactions among them. These interactions develop a synergistic co-evolutionary pressure under which gut AMPs are co-evolved to fight against MAR.
Natural gut resident bacteria, bacteria with acquired resistance genes, and acquired bacteria with resistance genes that do not typically colonize the gut are all included in the gut resistance reservoir [83]. Although it is uncommon, it is conceivable for resistance genes or virulence features to be transferred between pathogenic and non-pathogenic gut resident bacteria. The interesting question of how the resident gut bacteria and gut AMPs have maintained their efficiency through evolutionary timeframes is prompted by the growing issue of antibiotic-resistant bacteria. This question may have a partial explanation in the fact that there is a huge diversity of AMPs in the gut produced by intestinal epithelial cells as well as by healthy gut microbiota, decreasing the chance of combination resistance. Furthermore, since AMPs usually target bacterial cell walls and cell membranes that bacteria typically cannot modify without endangering their fitness, targeting crucial cell walls or cell membrane components likely also adds to the long-term efficiency of gut AMPs.
6. Antimicrobial Stewardship and Modulation of Gut AMPs as a Tool to Fight against Resistance Development
The rationalized use of antibiotics is an important aspect of fighting against antimicrobial resistance by maintaining gut homeostasis and reducing alterations to gut AMPs. The rationalized use of antibiotics includes the choice, dose, and duration of antibiotic therapy. It has been reported in several large meta-analysis studies that using antimicrobial stewardship programs resulted in a reduced number of infections with MAR organisms [84,85]. The type and spectrum of antibiotics employed are critical factors in the development of resistance in the targeted microorganisms. The majority of the commensal population is anaerobic; thus, inappropriate and extended usage of anti-anaerobic antibiotics has been linked to an increased risk of MAR [86,87]. It has been reported that the use of narrow-spectrum antibiotics in place of anti-anaerobic antibiotics is favorable to the human gut microbiota since fewer commensals are impacted [88,89]. Further, the duration of antibiotic therapy has a direct impact on gut microbiota composition as it has been reported that shorter antibiotic courses result in fewer microbial disturbances and quicker gut microbiota restoration [88,90]. Moreover, the right dose of antibiotic is very important as lower doses are linked with less chance of resistance gene development; however, lower doses for extended periods can also cause resistance [91,92].
Interestingly, gut AMPs have great promise as innovative therapeutic antibiotics because they do not easily develop bacterial resistance. The broad development of AMPs as medicines, however, has been hampered by several factors. First, AMPs can have relatively short half-lives because they are extremely sensitive to proteolytic breakdown by microbial and host enzymes. Second, many AMPs are harmful to the membranes of eukaryotic cells and display cytotoxicity.
Protein engineering techniques can be used to improve the bioavailability or efficacy of the AMPs because of their proteinaceous nature. It is possible to generate AMP versions that are resistant to enzymatic digestion. Also, using engineering peptidomimetics, new variants of AMPs could be generated with an altered number of charged amino acid residues with decreased hydrophobicity and cytotoxicity as well [93]. Additionally, the majority of AMPs kill bacteria by direct interaction with bacterial membranes. Interestingly, D-entantiomers of AMPs have longer half-lives and are just as effective at penetrating membranes as their natural L-entantiomers, so they can be used to improve the therapeutic efficacy of AMPs [94]. Further, packaging and delivering natural AMPs or their peptidomimetic analogs via nanoparticles can minimize non-specific cytotoxicity and improve stability with targeted bioavailability [95]. Additionally, novel AMPs should be employed for in vivo screening because the actual gut environment is completely different with the presence of different interactions with other commensals and their secreted AMPs which are already present in the gut. It is worth investigating the efficacy of new AMPs in the real dynamic gut environment against pathogenic bacteria or in conjunction with conventional antibiotics. Further new animal models with a controlled gut environment can be employed to check AMP efficacy in combination with antibiotics. Additionally, the gut has a diverse microbial ecology that differs for each individual. It is important to fully understand the gut microbial ecology for a detailed understanding of the interaction of gut AMPs with conventional antibiotics in the presence of other eukaryotic organisms including viruses, bacteriophages, and fungi. The interaction of gut AMPs with these diverse ecological community members individually or as a whole should be considered to understand their impact on gut AMP evolution and resistance development. Next-generation sequencing, transcriptomics, and gene expression analysis can further elucidate the mechanistic overview of complex gut environments that sheds light on unanswered questions and will further help in the development of a strategy to fight against resistance development.
7. Conclusions and Future Perspective
Combinatory use of AMPs produced by both host and gut microbiota with conventional antibiotics could result in synergistic actions in different ways. It has been predicted that every species contains a unique set of AMPs that are evolved to defend the host against the microorganisms they might encounter [96]. This phenomenon becomes more complex and functionally specific in the case of the gut. The human gut is inhabited by millions of commensals which constitute the specific set of bacteria for every individual that is further affected by dietary habits, environment, and many more factors. Interestingly, there is a highly competitive environment in the gut so gut microbes are known to produce AMPs with various biological activities including immunomodulatory activities. Along with AMPs produced by gut microbiota, there are multiple host AMPs secreted in the gut in the proximity of gut epithelium and gut microbiota. It is hypothesized that all the gut AMPs synergistically affect each other’s functions to drive complex gut functions such as regulation of gut homeostasis; however, the mechanisms of this are not fully understood. In addition to fighting against infectious pathogens, gut AMPs play an essential role in the regulation of bacterial symbionts and communities in the gut, thus maintaining a balance between health and pathogenic microbes [97]. Further, gut microbiota exhibit high intrinsic resistance to AMPs which suggests that gut AMPs could be a customizable tool to maintain healthy gut communities [98]. Additionally, recent accumulating pieces of evidence suggest a functional synergism among the different gut AMPs [99]. The gut synergism may also reduce the chances of resistance evolution. Further, the synergistic mechanisms of gut AMPs could be used effectively in combination with conventional antibiotics to combat MAR. Another factor is that the host regulates the gut AMPs synergistically in such a way that limits the chances of rapid resistance evolution. These synergistic strategies could be further used for the effective translation of AMPs alone or in combination with conventional antibiotics into therapeutic applications.
The human gut and AMP-producing intestinal epithelium constantly face a challenging dynamic microbial environment and also produce various antimicrobials for their survival that eventually affect the overall gut immune response including the efficacy of antibiotics during infection. To meet this challenge of the dynamic microbiome of the gut, epithelial cells also produce a wide variety of AMPs that quickly kill or inactivate bacteria, while a similar action is performed by the gut commensals to maintain the healthy gut environment which is called homeostasis. However, how the gut immune system differentiates between the healthy and pathogenic microbiota is still not well understood and remains a question of further research. On the other hand, in addition to this internal healthy equilibrium within the gut immune system, antibiotic treatment during infection creates another challenge for gut homeostasis. While both gut epithelium and commensals bear the adverse effects of antibiotics, gut AMPs have enough of a chance to interact with antibiotics, which affects the treatment efficacy as well (Table 2). However, it is not clear how AMPs interact with antibiotics and what is the response of AMP-producing gut epithelium and commensals in this dynamic complex gut environment. The emerging picture is that epithelial AMPs influence the structure and location of gut commensals in addition to protecting against pathogen colonization and invasion in synergism with the AMPs produced by commensals. Overall, gut AMPs are evolved for their antimicrobial action, efficacy, and spectrum under synergistic co-evolution with host immunity and commensals, along with interactions with other AMPs and conventional antibiotics (Figure 2).
Finally, MAR resistance is rapidly growing while the discovery and availability of new antimicrobials are slow which generates an urgent demand for new antimicrobials along with a fully elucidated mechanism of resistance to overcome this crisis. At this point, the dissection of the gut microbiome as an antimicrobial resistance reservoir is much needed. This can be achieved by clinical and translational studies exploring the interaction of gut microbiome and gut AMPs within the gut microbial ecology and with conventional antibiotics. Functional metagenomic studies might be very helpful in identifying the uncultivable gut microbes and their role in resistance development and evolution.
8. Unanswered Questions about Gut Microbiota and Gut AMPs
What makes the gut microbiome healthy and what are the deciding bio-markers?
What is the genetic machinery that regulates the production of gut AMPs?
How do gut AMPs play a role in resistance development?
Could diet help in the fight against resistance by manipulating gut microbiota? How?
How do gut AMPs regulate the immune response to fight against resistance?
How to reconstruct the gut microbiome and gut AMPome to counter antibiotic resistance?
Acknowledgments
The authors are thankful to the Department of Radiation Oncology, University of Missouri, Columbia, and the Department of Biotechnology, Indian Institute of Technology, Kharagpur for providing the space and necessary facilities for this work.
Author Contributions
P.B. conceptualized and wrote the manuscript and prepared the illustrations. P.B. and S.M.M. edited and proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Murray C.J., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ventola C.L. The antibiotic resistance crisis: Causes and threats. Pharm. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Mwangi J., Hao X., Lai R., Zhang Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019;40:488–505. doi: 10.24272/j.issn.2095-8137.2019.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baindara P., Chaudhry V., Mittal G., Liao L.M., Matos C.O., Khatri N., Franco O.L., Patil P.B., Korpole S. Characterization of the antimicrobial peptide penisin, a class Ia novel lantibiotic from Paenibacillus sp. strain A3. Antimicrob. Agents Chemother. 2016;60:580–591. doi: 10.1128/AAC.01813-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josef J. Drug repurposing to overcome microbial resistance. Drug Discov. Today. 2022;27:2028–2041. doi: 10.1016/j.drudis.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Deslouches B., Montelaro R.C., Urish K.L., Di Y.P. Engineered cationic antimicrobial peptides (eCAPs) to combat multidrug-resistant bacteria. Pharmaceutics. 2020;12:501. doi: 10.3390/pharmaceutics12060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baindara P., Mandal S.M. Antimicrobial Peptides and Vaccine Development to Control Multi-drug Resistant Bacteria. Protein Pept. Lett. 2019;26:324–331. doi: 10.2174/0929866526666190228162751. [DOI] [PubMed] [Google Scholar]
- 8.Mathur H., Field D., Rea M.C., Cotter P.D., Hill C., Ross R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes. 2018;4:9. doi: 10.1038/s41522-018-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S., Thacker P., Watford M., Qiao S. Functions of Antimicrobial Peptides in Gut Homeostasis. Curr. Protein Pept. Sci. 2015;16:582–591. doi: 10.2174/1389203716666150630135847. [DOI] [PubMed] [Google Scholar]
- 10.Lewies A., Du Plessis L.H., Wentzel J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins. 2019;11:370–381. doi: 10.1007/s12602-018-9465-0. [DOI] [PubMed] [Google Scholar]
- 11.Costa F., Teixeira C., Gomes P., Martins M.C.L. Advances in Experimental Medicine and Biology. Volume 1117. Springer; Berlin/Heidelberg, Germany: 2019. Clinical application of AMPs; pp. 281–298. [DOI] [PubMed] [Google Scholar]
- 12.Baindara P., Ghosh A.K., Mandal S.M. Coevolution of Resistance Against Antimicrobial Peptides. Microb. Drug Resist. 2020;26:880–899. doi: 10.1089/mdr.2019.0291. [DOI] [PubMed] [Google Scholar]
- 13.El Shazely B., Yu G., Johnston P.R., Rolff J. Resistance Evolution Against Antimicrobial Peptides in Staphylococcus aureus Alters Pharmacodynamics Beyond the MIC. Front. Microbiol. 2020;11:103. doi: 10.3389/fmicb.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinata R., Baindara P. Laterosporulin25: A probiotically produced, novel defensin-like bacteriocin and its immunogenic properties. Int. Immunopharmacol. 2023;121:110500. doi: 10.1016/j.intimp.2023.110500. [DOI] [PubMed] [Google Scholar]
- 15.Francino M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafii F., Sutherland J.B., Cerniglia C.E. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther. Clin. Risk Manag. 2008;4:1343–1357. doi: 10.2147/TCRM.S4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid M.U., Weintraub A., Nord C.E. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe. 2012;18:249–253. doi: 10.1016/j.anaerobe.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov I.I., de Frutos R.L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wlodarska M., Willing B., Keeney K.M., Menendez A., Bergstrom K.S., Gill N., Russell S.L., Vallance B.A., Finlay B.B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011;79:1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood C., Morrow A.L., Lagomarcino A.J., Altaye M., Taft D.H., Yu Z., Newburg D.S., Ward D.V., Schibler K.R. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of enterobacter. J. Pediatr. 2014;165:23–29. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.-Y., Tsolis R.M., Bäumler A.J. The microbiome and gut homeostasis. Science. 2022;377:eabp9960. doi: 10.1126/science.abp9960. [DOI] [PubMed] [Google Scholar]
- 23.Cotter P.D., Ross R.P., Hill C. Bacteriocins-a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 24.Donia M.S., Cimermancic P., Schulze C.J., Wieland Brown L.C., Martin J., Mitreva M., Clardy J., Linington R.G., Fischbach M.A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158:1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corr S.C., Li Y., Riedel C.U., O’Toole P.W., Hill C., Gahan C.G.M. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea M.C., Sit C.S., Clayton E., O’Connor P.M., Whittal R.M., Zheng J., Vederas J.C., Ross R.P., Hill C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. USA. 2010;107:9352–9357. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmore M.S., Lebreton F., van Schaik W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 2013;16:10–16. doi: 10.1016/j.mib.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kommineni S., Bretl D.J., Lam V., Chakraborty R., Hayward M., Simpson P., Cao Y., Bousounis P., Kristich C.J., Salzman N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature. 2015;526:719–722. doi: 10.1038/nature15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sassone-Corsi M., Nuccio S.P., Liu H., Hernandez D., Vu C.T., Takahashi A.A., Edwards R.A., Raffatellu M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540:280–283. doi: 10.1038/nature20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riboulet-Bisson E., Sturme M.H.J., Jeffery I.B., O’Donnell M.M., Neville B.A., Forde B.M., Claesson M.J., Harris H., Gardiner G.E., Casey P.G., et al. Effect of lactobacillus salivarius bacteriocin ABP118 on the mouse and pig intestinal microbiota. PLoS ONE. 2012;7:e31113. doi: 10.1371/journal.pone.0031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilinskaya O.N., Ulyanova V.V., Yarullina D.R., Gataullin I.G. Secretome of Intestinal bacilli: A natural guard against pathologies. Front. Microbiol. 2017;8:1666. doi: 10.3389/fmicb.2017.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’ Connor P.M., O’ Shea E.F., Cotter P.D., Hill C., Ross R.P. The potency of the broad spectrum bacteriocin, bactofencin A, against staphylococci is highly dependent on primary structure, N-terminal charge and disulphide formation. Sci. Rep. 2018;8:11833. doi: 10.1038/s41598-018-30271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang F., Teng K., Liu Y., Cao Y., Wang T., Ma C., Zhang J., Zhong J. Bacteriocins: Potential for Human Health. Oxid. Med. Cell. Longev. 2021;2021:5518825. doi: 10.1155/2021/5518825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belfiore C., Castellano P., Vignolo G. Reduction of Escherichia coli population following treatment with bacteriocins from lactic acid bacteria and chelators. Food Microbiol. 2007;24:223–229. doi: 10.1016/j.fm.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Rea M.C., Clayton E., O’Connor P.M., Shanahan F., Kiely B., Ross R.P., Hill C. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J. Med. Microbiol. 2007;56:940–946. doi: 10.1099/jmm.0.47085-0. [DOI] [PubMed] [Google Scholar]
- 36.Borrero J., Brede D.A., Skaugen M., Diep D.B., Herranz C., Nes I.F., Cintas L.M., Hernández P.E. Characterization of garvicin ML, a novel circular bacteriocin produced by lactococcus garvieae DCC43, isolated from mallard ducks (Anas platyrhynchos) Appl. Environ. Microbiol. 2011;77:369–373. doi: 10.1128/AEM.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millette M., Cornut G., Dupont C., Shareck F., Archambault D., Lacroix M. Capacity of human nisin- and pediocin-producing lactic acid bacteria to reduce intestinal colonization by vancomycin-resistant enterococci. Appl. Environ. Microbiol. 2008;74:1997–2003. doi: 10.1128/AEM.02150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Kwaadsteniet M., Doeschate K.T., Dicks L.M.T. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett. Appl. Microbiol. 2009;48:65–70. doi: 10.1111/j.1472-765X.2008.02488.x. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein B.P., Wei J., Greenberg K., Novick R. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 1998;42:277–278. doi: 10.1093/jac/42.2.277. [DOI] [PubMed] [Google Scholar]
- 40.Norouzi Z., Salimi A., Halabian R., Fahimi H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb. Pathog. 2018;123:183–189. doi: 10.1016/j.micpath.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Dabour N., Zihler A., Kheadr E., Lacroix C., Fliss I. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 2009;133:225–233. doi: 10.1016/j.ijfoodmicro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Salvucci E., Saavedra L., Hebert E.M., Haro C., Sesma F. Enterocin CRL35 inhibits Listeria monocytogenes in a murine model. Foodborne Pathog. Dis. 2012;9:68–74. doi: 10.1089/fpd.2011.0972. [DOI] [PubMed] [Google Scholar]
- 43.Birri D.J., Brede D.A., Forberg T., Holo H., Nes I.F. Molecular and genetic characterization of a novel bacteriocin locus in Enterococcus avium isolates from infants. Appl. Environ. Microbiol. 2010;76:483–492. doi: 10.1128/AEM.01597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Kwaadsteniet M., Fraser T., Van Reenen C.A., Dicks L.M.T. Bacteriocin T8, a novel class IIa sec-dependent bacteriocin produced by Enterococcus faecium T8, isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl. Environ. Microbiol. 2006;72:4761–4766. doi: 10.1128/AEM.00436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin-Visscher L.A., van Belkum M.J., Garneau-Tsodikova S., Whittal R.M., Zheng J., McMullen L.M., Vederas J.C. Isolation and characterization of carnocyclin a, a novel circular bacteriocin produced by Carnobacterium maltaromaticum UAL307. Appl. Environ. Microbiol. 2008;74:4756–4763. doi: 10.1128/AEM.00817-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang J.Y., Chang H.C. Growth Inhibition of Foodborne Pathogens by Kimchi Prepared with Bacteriocin-Producing Starter Culture. J. Food Sci. 2011;76:M72–M78. doi: 10.1111/j.1750-3841.2010.01965.x. [DOI] [PubMed] [Google Scholar]
- 47.Dey D., Ema T.I., Biswas P., Aktar S., Islam S., Rinik U.R., Firoz M., Ahmed S.Z., Al Azad S., Rahman A., et al. Antiviral effects of bacteriocin against animal-to-human transmittable mutated SARS-CoV-2: A systematic review. Front. Agric. Sci. Eng. 2021;8:603–622. doi: 10.15302/J-FASE-2021397. [DOI] [Google Scholar]
- 48.Filipp D., Brabec T., Vobořil M., Dobeš J. Enteric α-defensins on the verge of intestinal immune tolerance and inflammation. Semin. Cell Dev. Biol. 2019;88:138–146. doi: 10.1016/j.semcdb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Phadke S.M., Deslouches B., Hileman S.E., Montelaro R.C., Wiesenfeld H.C., Mietzner T.A. Antimicrobial peptides in mucosal secretions: The importance of local secretions in mitigating infection. J. Nutr. 2005;135:1289–1293. doi: 10.1093/jn/135.5.1289. [DOI] [PubMed] [Google Scholar]
- 50.Dutta P., Das S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015;16:99–129. doi: 10.2174/1568026615666150703121819. [DOI] [PubMed] [Google Scholar]
- 51.Ayabe T., Satchell D.P., Wilson C.L., Parks W.C., Selsted M.E., Ouellette A.J. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 52.Menendez A., Willing B.P., Montero M., Wlodarska M., So C.C., Bhinder G., Vallance B.A., Finlay B.B. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal paneth cell α-defensins. J. Innate Immun. 2013;5:39–49. doi: 10.1159/000341630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzman N.H., Hung K., Haribhai D., Chu H., Karlsson-Sjöberg J., Amir E., Teggatz P., Barman M., Hayward M., Eastwood D., et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fusco A., Savio V., Cammarota M., Alfano A., Schiraldi C., Donnarumma G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017;2017:6976935. doi: 10.1155/2017/6976935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi H., Hu W., Chen S., Lu Z., Wang Y. Cathelicidin-WA Improves Intestinal Epithelial Barrier Function and Enhances Host Defense against Enterohemorrhagic Escherichia coli O157:H7 Infection. J. Immunol. 2017;198:1696–1705. doi: 10.4049/jimmunol.1601221. [DOI] [PubMed] [Google Scholar]
- 56.Iimura M., Gallo R.L., Hase K., Miyamoto Y., Eckmann L., Kagnoff M.F. Cathelicidin Mediates Innate Intestinal Defense against Colonization with Epithelial Adherent Bacterial Pathogens. J. Immunol. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 57.Chromek M., Arvidsson I., Karpman D. The Antimicrobial Peptide Cathelicidin Protects Mice from Escherichia coli O157:H7-Mediated Disease. PLoS ONE. 2012;7:e46476. doi: 10.1371/journal.pone.0046476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimura T., McLean M.H., Dzutsev A.K., Yao X., Chen K., Huang J., Gong W., Zhou J., Xiang Y., Badger J.H., et al. The Antimicrobial Peptide CRAMP Is Essential for Colon Homeostasis by Maintaining Microbiota Balance. J. Immunol. 2018;200:2174–2185. doi: 10.4049/jimmunol.1602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIII gamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandl K., Plitas G., Schnabl B., DeMatteo R.P., Pamer E.G. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ju T., Shoblak Y., Gao Y., Yang K., Fouhse J., Finlay B.B., So Y.W., Stothard P., Willing B.P. Initial gut microbial composition as a key factor driving host response to antibiotic treatment, as exemplified by the presence or absence of commensal Escherichia coli. Appl. Environ. Microbiol. 2017;83:e01107-17. doi: 10.1128/AEM.01107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baindara P., Singh N., Ranjan M., Nallabelli N., Chaudhry V., Pathania G.L., Sharma N., Kumar A., Patil P.B., Korpole S. Laterosporulin10: A novel defensin like class iid bacteriocin from brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiology. 2016;162:1286–1299. doi: 10.1099/mic.0.000316. [DOI] [PubMed] [Google Scholar]
- 63.Naghmouchi K., Le Lay C., Baah J., Drider D. Antibiotic and antimicrobial peptide combinations: Synergistic inhibition of Pseudomonas fluorescens and antibiotic-resistant variants. Res. Microbiol. 2012;163:101–108. doi: 10.1016/j.resmic.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Tong Z., Zhang Y., Ling J., Ma J., Huang L., Zhang L. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE. 2014;9:e89209. doi: 10.1371/journal.pone.0089209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebel G., Piché F., Frenette M., Gottschalk M., Grenier D. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides. 2013;50:19–23. doi: 10.1016/j.peptides.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Cavera V.L., Volski A., Chikindas M.L. The Natural Antimicrobial Subtilosin A Synergizes with Lauramide Arginine Ethyl Ester (LAE), ε-Poly-l-lysine (Polylysine), Clindamycin Phosphate and Metronidazole, Against the Vaginal Pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins. 2015;7:164–171. doi: 10.1007/s12602-014-9183-1. [DOI] [PubMed] [Google Scholar]
- 67.Nuding S., Frasch T., Schaller M., Stange E.F., Zabel L.T. Synergistic effects of antimicrobial peptides and antibiotics against clostridium difficile. Antimicrob. Agents Chemother. 2014;58:5719–5725. doi: 10.1128/AAC.02542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rishi P., Preet S., Bharrhan S., Verma I. In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob. Agents Chemother. 2011;55:4176–4182. doi: 10.1128/AAC.00273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalita A., Verma I., Khuller G.K. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J. Infect. Dis. 2004;190:1476–1480. doi: 10.1086/424463. [DOI] [PubMed] [Google Scholar]
- 70.Rajasekaran G., Kim E.Y., Shin S.Y. LL-37-derived membrane-active FK-13 analogs possessing cell selectivity, anti-biofilm activity and synergy with chloramphenicol and anti-inflammatory activity. Biochim. Biophys. Acta Biomembr. 2017;1859:722–733. doi: 10.1016/j.bbamem.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 71.Lin L., Nonejuie P., Munguia J., Hollands A., Olson J., Dam Q., Kumaraswamy M., Rivera H., Corriden R., Rohde M., et al. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine. 2015;2:690–698. doi: 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruden S., Rieder A., Chis Ster I., Schwartz T., Mikut R., Hilpert K. Synergy Pattern of Short Cationic Antimicrobial Peptides against Multidrug-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2019;10:2740. doi: 10.3389/fmicb.2019.02740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrmann G., Yang L., Wu H., Song Z., Wang H., Høiby N., Ulrich M., Molin S., Riethmüller J., Döring G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010;202:1585–1592. doi: 10.1086/656788. [DOI] [PubMed] [Google Scholar]
- 74.Brumfitt W., Salton M.R.J., Hamilton-Miller J.M.T. Nisin, alone and combined with peptidoglycan-modulating antibiotics: Activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 2002;50:731–734. doi: 10.1093/jac/dkf190. [DOI] [PubMed] [Google Scholar]
- 75.Giacometti A., Cirioni O., Barchiesi F., Fortuna M., Scalise G. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1999;44:641–645. doi: 10.1093/jac/44.5.641. [DOI] [PubMed] [Google Scholar]
- 76.Draper L.A., Cotter P.D., Hill C., Ross R.P. The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol. 2013;13:212. doi: 10.1186/1471-2180-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathur H., O’Connor P.M., Hill C., Cotter P.D., Ross R.P. Analysis of anti-clostridium difficile activity of thuricin CD, vancomycin, metronidazole, ramoplanin, and actagardine, both singly and in paired combinations. Antimicrob. Agents Chemother. 2013;57:2882–2886. doi: 10.1128/AAC.00261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lobos O., Padilla A., Padilla C. In vitro antimicrobial effect of bacteriocin PsVP-10 in combination with chlorhexidine and triclosan against Streptococcus mutans and Streptococcus sobrinus strains. Arch. Oral Biol. 2009;54:230–234. doi: 10.1016/j.archoralbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 79.Sharma A., Srivastava S. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol. 2014;118:264–275. doi: 10.1016/j.funbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Larsen J., Raisen C.L., Ba X., Sadgrove N.J., Padilla-González G.F., Simmonds M.S.J., Loncaric I., Kerschner H., Apfalter P., Hartl R., et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature. 2022;602:135–141. doi: 10.1038/s41586-021-04265-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Toole R.F., Leong K.W.C., Cumming V., Van Hal S.J. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. 2023;174:104046. doi: 10.1016/j.resmic.2023.104046. [DOI] [PubMed] [Google Scholar]
- 82.Forslund K., Sunagawa S., Kultima J.R., Mende D.R., Arumugam M., Typas A., Bork P. Country-specific antibiotic use practices impact the human gut resistome. Genome Res. 2013;23:1163–1169. doi: 10.1101/gr.155465.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anthony W.E., Burnham C.A.D., Dantas G., Kwon J.H. The gut microbiome as a reservoir for antimicrobial resistance. J. Infect. Dis. 2021;223:S209–S213. doi: 10.1093/infdis/jiaa497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baur D., Gladstone B.P., Burkert F., Carrara E., Foschi F., Döbele S., Tacconelli E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017;17:990–1001. doi: 10.1016/S1473-3099(17)30325-0. [DOI] [PubMed] [Google Scholar]
- 85.Ya K.Z., Win P.T.N., Bielicki J., Lambiris M., Fink G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2023;6:E2253806. doi: 10.1001/jamanetworkopen.2022.53806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chanderraj R., Baker J.M., Kay S.G., Brown C.A., Hinkle K.J., Fergle D.J., McDonald R.A., Falkowski N.R., Metcalf J.D., Kaye K.S., et al. In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur. Respir. J. 2023;61:2200910. doi: 10.1183/13993003.00910-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhalla A., Pultz N.J., Ray A.J., Hoyen C.K., Eckstein E.C., Donskey C.J. Antianaerobic Antibiotic Therapy Promotes Overgrowth of Antibiotic-Resistant, Gram-Negative Bacilli and Vancomycin-Resistant Enterococci in the Stool of Colonized Patients. Infect. Control Hosp. Epidemiol. 2003;24:644–649. doi: 10.1086/502267. [DOI] [PubMed] [Google Scholar]
- 88.Mitchell B.G., Hall L., White N., Barnett A.G., Halton K., Paterson D.L., Riley T.V., Gardner A., Page K., Farrington A., et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): A multicentre, randomised trial. Lancet Infect. Dis. 2019;19:410–418. doi: 10.1016/S1473-3099(18)30714-X. [DOI] [PubMed] [Google Scholar]
- 89.Shahi F., Redeker K., Chong J. Rethinking antimicrobial stewardship paradigms in the context of the gut microbiome. JAC—Antimicrob. Resist. 2019;1:dlz015. doi: 10.1093/jacamr/dlz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leo S., Lazarevic V., von Dach E., Kaiser L., Prendki V., Schrenzel J., Huttner B.D., Huttner A. Effects of antibiotic duration on the intestinal microbiota and resistome: The PIRATE RESISTANCE project, a cohort study nested within a randomized trial. EBioMedicine. 2021;71:103566. doi: 10.1016/j.ebiom.2021.103566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L., Huang Y., Zhou Y., Buckley T., Wang H.H. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob. Agents Chemother. 2013;57:3659–3666. doi: 10.1128/AAC.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wistrand-Yuen E., Knopp M., Hjort K., Koskiniemi S., Berg O.G., Andersson D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018;9:1599. doi: 10.1038/s41467-018-04059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mojsoska B., Jenssen H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals. 2015;8:366–415. doi: 10.3390/ph8030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu J., Xu H., Xia J., Ma J., Xu J., Li Y., Feng J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides with Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020;11:563030. doi: 10.3389/fmicb.2020.563030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fadaka A.O., Sibuyi N.R.S., Madiehe A.M., Meyer M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics. 2021;13:1795. doi: 10.3390/pharmaceutics13111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 97.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 98.Cullen T.W., Schofield W.B., Barry N.A., Putnam E.E., Rundell E.A., Trent M.S., Degnan P.H., Booth C.J., Yu H., Goodman A.L. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 2015;347:170–175. doi: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan H., Hancock R.E.W. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 2001;45:1558–1560. doi: 10.1128/AAC.45.5.1558-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]