Abstract
Aims
Prediction of adverse events in mid-term follow-up after transcatheter aortic valve implantation (TAVI) is challenging. We sought to develop and validate a machine learning model for prediction of 1-year all-cause mortality in patients who underwent TAVI and were discharged following the index procedure.
Methods and results
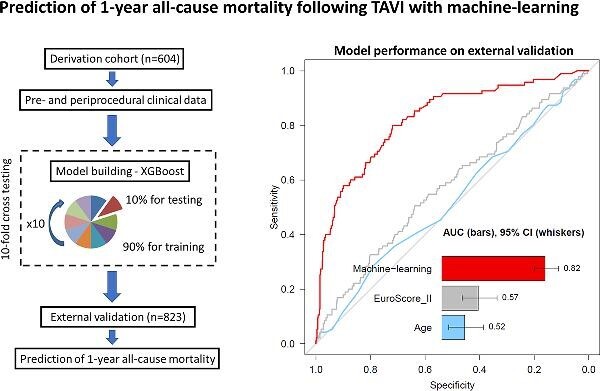
The model was developed on data of patients who underwent TAVI at a high-volume centre between January 2013 and March 2019. Machine learning by extreme gradient boosting was trained and tested with repeated 10-fold hold-out testing using 34 pre- and 25 peri-procedural clinical variables. External validation was performed on unseen data from two other independent high-volume TAVI centres. Six hundred four patients (43% men, 81 ± 5 years old, EuroSCORE II 4.8 [3.0–6.3]%) in the derivation and 823 patients (46% men, 82 ± 5 years old, EuroSCORE II 4.7 [2.9–6.0]%) in the validation cohort underwent TAVI and were discharged home following the index procedure. Over the 12 months of follow-up, 68 (11%) and 95 (12%) subjects died in the derivation and validation cohorts, respectively. In external validation, the machine learning model had an area under the receiver-operator curve of 0.82 (0.78–0.87) for prediction of 1-year all-cause mortality following hospital discharge after TAVI, which was superior to pre- and peri-procedural clinical variables including age 0.52 (0.46–0.59) and the EuroSCORE II 0.57 (0.51–0.64), P < 0.001 for a difference.
Conclusion
Machine learning based on readily available clinical data allows accurate prediction of 1-year all-cause mortality following a successful TAVI.
Keywords: Artificial intelligence, Machine learning, Transcatheter aortic valve implantation, Aortic stenosis
Graphical Abstract
Graphical Abstract.
Introduction
Transcatheter aortic valve implantation (TAVI) has revolutionized the management of severe, symptomatic aortic valve stenosis.1–4 While according to recent nationwide registry data, TAVI outcomes are improving over time across a range of important metrics, the 1-year mortality following implantation remains substantial at 10–12% in 2020.5–7 This relatively high event rate can be largely attributed to the advanced age, frailty, and competing cardiovascular as well as non-cardiovascular risk, which all jointly affect TAVI recipients. The high comorbidity burden of patients undergoing TAVI makes prediction of outcome following a successful bioprosthesis implantation challenging. While several methods for prediction of TAVI outcomes have been proposed, these efforts have largely focused on prediction of in-hospital and/or 30-day mortality, and their performance remained limited.8–11 Given the extensive diagnostic work-up that precedes TAVI, it is plausible that the wealth of pre- and peri-procedural data could be leveraged for robust risk stratification.
Artificial intelligence with machine learning has emerged as a powerful tool for combining several weak predictors in a single model for enhanced prediction of adverse outcomes.12,13 Recently, gradient boosting algorithms have been shown to enhance risk stratification across a wide range of diseases and clinical scenarios providing patient-specific prediction beyond conventional risk scores.14–16 In this study, we leveraged a state-of-the-art gradient boosting algorithm to develop a prediction model for all-cause mortality in the year following a successful TAVI procedure and validated its performance on external datasets from independent sites.
Methods
Study design
The study population included three cohorts of consecutive TAVI recipients from tertiary high-volume centres (each performing >100 procedures annually) who underwent valve implantation between January 2013 and March 2019. All patients underwent a comprehensive baseline clinical assessment with evaluation of their cardiovascular risk factor profile, including calculation of risk scores (European System for Cardiac Operative Risk Evaluation—EuroSCORE II, the France II, and OBSERVANT scores).8,9 Only subjects who underwent a successful TAVI and were discharged home following the index procedure were included. Data from the Institute of Cardiology, Warsaw served as the derivation cohort for the machine learning model, which was then further tested on unseen external datasets from the Medical University of Warsaw and the Cardiovascular Institute, Hospital Clinico San Carlos, Madrid (validation cohort) Figure (1). This paper was written according to recommendations in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.17 The study was conducted with the approval of the local research ethics committee at the National Institute of Cardiology, Warsaw, Poland (registration number IK.NPIA.0021.1.1954/22) and in accordance with the Declaration of Helsinki.
Figure 1.
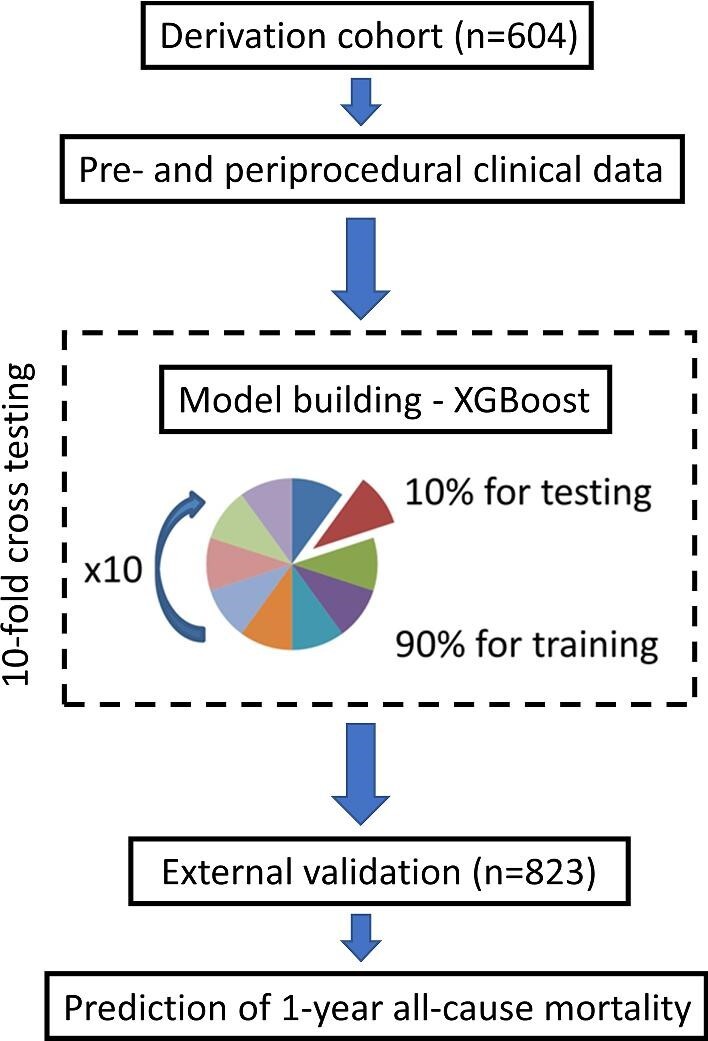
Study design. Our machine learning model was developed on 604 patients from the derivation cohort using 10-fold repeated cross-testing. Subsequently, the model was validated on unseen data from the validation cohort (n = 823).
Clinical follow-up
The primary endpoint of the study was 1-year all-cause mortality following hospital discharge after a successful TAVI procedure. Outcome information was obtained from local and national healthcare record systems. Categorization of these outcomes was performed blinded to the clinical patient data.
Machine learning
Machine learning was used to derive a joint probabilistic score that could inform the physician on the risk of 1-year all-cause mortality after TAVI and therefore facilitate planning post-discharge care and surveillance. We have therefore excluded patients who died during the index hospitalization from the analysis. The score was based on 34 pre- and 25 peri-procedural clinical variables: baseline characteristics, cardiovascular risk factors (comorbidities), echocardiography- and blood-derived biomarkers, as well as procedural aspects (access, radiation, and complications), and pre-discharge echocardiography and blood tests (Supplementary material online, Table 1).
Model building
XGBoost is a recent implementation of a gradient boosting algorithm, which iteratively trains a set of weak predictors (simple decision trees) using a given set of patient data, to build a combined strong classifier to identify an outcome.14–16 For every patient, the XGBoost algorithm computes an individualized probability of outcome, considering all input variables. All variables utilized in the machine learning modelling are presented in Supplementary material online, Table 1. For optimal model performance, we have performed hypertuning of XGBoost parameters (Supplementary material online, Methods). The model configuration providing the best prediction accuracy was selected.
Internal testing
To avoid biased results, limit overfitting, and ascertain generalizability of our model using the derivation cohort, we tested the model using repeated 10-fold cross-testing, which separates training and testing data.18 The dataset was randomly split into 10 folds with similar all-cause mortality rates in each fold (stratified 10 folds). Ten models were created each from 90% of the data, and each tested in a held-out test sample (10% of the data). These 10 held-out samples containing non-overlapping test results were subsequently concatenated to evaluate the average performance of XGBoost in unseen data.
External testing
To further validate the generalizability of this approach, we have built the model from all the data used for the internal testing. Subsequently, we have conducted external validation of this model on real-world data from two independent high-volume TAVI centres (validation cohort) (Figure 1).
Feature importance
To elucidate the influence of each of the variables included in the machine learning model, we provided machine learning feature importance scores. Importance is the relative amount that each attribute improves the XGBoost performance measure (similar to information gain). The variable importance was determined directly from the XGBoost model separately in each fold and returned from the XGBoost model for each variable.19
Individualized explainability
Further in this study, we provide a description of individualized predictions made by the algorithm.14,20 This internal XGBoost function allows identification of important patient-specific features and the role of the feature in the predicted score for the specific patient and may facilitate the clinical acceptance of the artificial intelligence approach. The individual explanation can be achieved by analysis of the specific path a subject takes in the model as in each decision stump (or split) of the model, the individual lands in one of two leaves. Each leaf is associated with a weight: one leaf decreases the risk of the event happening, and the other one increases the risk. Ultimately, this information can be graphically presented with waterfall plots.
Statistical analysis
We assessed the distribution of data with the Shapiro–Wilk test. Continuous parametric variables were expressed as mean ± standard deviation, and non-parametric data were presented as median (interquartile interval). Fisher’s exact test or χ2 test was used for the analysis of categorical variables. The performance of machine learning models and single clinical characteristics in predicting all-cause mortality was assessed using receiver-operator characteristic (ROC) analysis, and the area under the curve (AUC) values were compared with the DeLong test.21 To evaluate the accuracy of predictors, we have also quantified the sensitivity, specificity, positive and negative predictive values of each clinical variable. For continuous variables, the Youden index was employed to define the optimal thresholds. Statistical analysis was performed with SPSS version 24 (IBM SPSS Statistics for Windows, Version 24.0, Armonk, NY: IBM Corp.) and R studio and R software version 4.01 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P < 0.05 was considered statistically significant.
Results
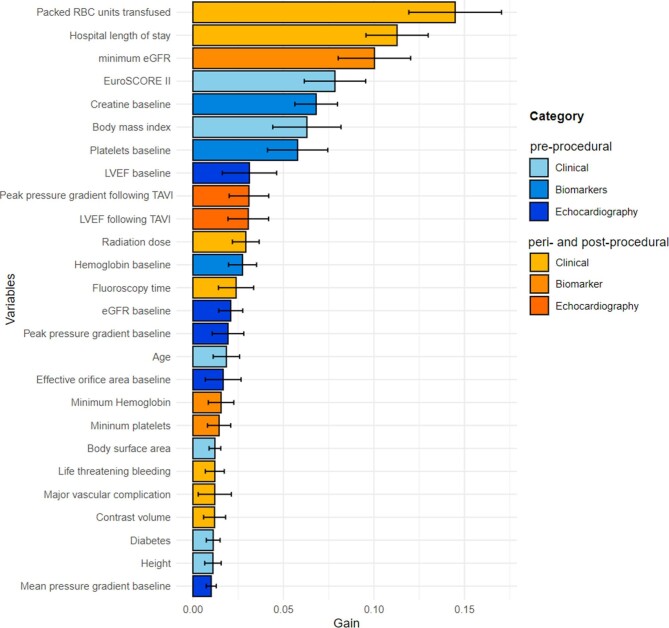
In the derivation cohort, 604 patients (43% men, 81 ± 5.1 years old, EuroSCORE II 4.8 [3.0–6.3]%) underwent TAVI and were discharged home following the index procedure. Over 12 months following the index procedure, 68 (11%) patients died. Baseline demographic, clinical, echocardiographic, and procedural characteristics of the study population are listed in Table1. Only a few clinical variables emerged as predictors of 1-year all-cause mortality following hospital discharge after a successful TAVI. These included baseline kidney function, platelet levels, the lowest post-procedural kidney function, left ventricular ejection fraction, length of stay in the hospital after TAVI, and the amount of packed red blood cells transfused (Table 2). The predictive performance of these variables in isolation was, however, limited with the highest AUC (95% confidence interval) of 0.67 (0.59–0.74) for kidney function following TAVI and 0.64 (0.56–0.72) for the left ventricular ejection fraction on post-procedural echocardiography. Importantly, neither patient age nor the EuroSCORE II were significant predictors of all-cause mortality: AUC 0.51 (0.44–0.57) and 0.56 (0.49–0.63), respectively (P = 0.83 and P = 0.08). The overall variable importance for the classification of all-cause mortality is depicted in Figure 2. While the number of packed red blood cell units transfused, the hospital length of stay, and the lowest estimated glomerular filtration rate were the top predictors, baseline blood biomarkers (creatine and platelet levels), and echocardiographic findings (both baseline and post-procedural) were also among the variables that had the greatest contribution to the machine learning model (Figure 2).
Table 1.
Baseline characteristics of study participants
| Validation cohort (n = 823) | ||||
|---|---|---|---|---|
| Derivation cohort (n = 604) | Medical University of Warsaw | Hospital Clinico San Carlos | P-value | |
| Age, years | 82 [77–86] | 81 [76–84] | 83 [79–83] | 0.37 |
| Females, n | 345 (57%) | 108 (52%) | 337 (55%) | 0.24 |
| Weight, kg | 73.5 ± 16 | 75 ± 15 | 71 ± 15 | 0.27 |
| Body mass index | 27.1 ± 5.3 | 27.2 ± 4.5 | 27.9 ± 5.2 | 0.41 |
| Bicuspid aortic valve | 53 (9%) | 17 (8%) | 43 (7%) | 0.50 |
| EuroScore II | 4.8 [3.0–6.3] | 5.1 [3.3–7.4] | 4.5 [2.9–7.3] | 0.29 |
| Comorbidities and past medical history | ||||
| Diabetes | 226 (37%) | 72 (35%) | 208 (34%) | 0.53 |
| Atrial fibrillation | 206 (34%) | 79 (38%) | 248 (40%) | 0.31 |
| History of ACS | 130 (21.5%) | 35 (17%) | 97 (16%) | 0.25 |
| History of PCI | 175 (29%) | 74 (36%) | 149 (24%) | 0.19 |
| History of CABG | 77 (13%) | 22 (11%) | 63 (10%) | 0.22 |
| History of valve surgery | 24 (4%) | 10 (5%) | 31 (5%) | 0.28 |
| History of pacemaker implantation | 86 (14%) | 36 (17%) | 82 (13%) | 0.33 |
| History of a cerebrovascular accident | 77 (13%) | 21 (10%) | 57 (9%) | 0.23 |
| Baseline biomarkers | ||||
| Creatinine, mg/dL | 1.1 [0.9–1.4] | 1.1 [0.8–1.5] | 1.0 [0.8–1.3] | 0.51 |
| eGFR, mL/m2 | 55 [42–70] | 54 [36–79] | 59 [44–74] | 0.38 |
| Haemoglobin, g/dL | 12.4 [11.2–13.3] | 11.4 [10.3–12.8] | 12.3 [11.0–13.3] | 0.43 |
| Platelets, n/dL | 174 [138–219] | 193 [150–231] | 187 [156–230] | 0.32 |
| Baseline echocardiography | ||||
| Left ventricular ejection fraction, % | 55 [50–65] | 58 [46–64] | 55 [50–65] | 0.26 |
| Effective orifice area, cm2 | 0.6 [0.5–0.8] | 0.7 [0.5–0.9] | 0.6 [0.5–0.8] | 0.36 |
| Peak transvalvular pressure gradient, mmHg | 86 [70–104] | 77 [61–96] | 76 [61–90] | 0.29 |
| Mean transvalvular pressure gradient, mmHg | 49 [39–62] | 43 [34–51] | 44 [38–55] | 0.27 |
| Procedure—valve implantation | ||||
| Contrast media volume, mL | 190 [150–200] | 200 [150–250] | 164 [137–200] | 0.38 |
| Fluoroscopy time, min | 24 [20–35] | 31 [21–45] | 25 [18–31] | 0.23 |
| Radiation dose, mGy | 1051 [608– 1737] | 1251 [810–2004] | 1134 [682–1680] | 0.28 |
| Peri- and post-procedural outcomes | ||||
| Major vascular complication | 30 (5%) | 12 (6%) | 25 (4%) | 0.27 |
| Minor vascular complication | 57 (9%) | 10 (5%) | 69 (11%) | 0.034 |
| Life threatening bleeding | 24 (4%) | 10 (5%) | 22 (4%) | 0.31 |
| Major bleeding | 80 (13%) | 21 (10%) | 27 (4%) | 0.001 |
| Minor bleeding | 86 (14%) | 19 (9%) | 95 (15%) | 0.11 |
| Total RBC concentrate transfused, units | 0 [0–1] | 0 [0–1] | 0 [0–1] | 0.38 |
| Peri-procedural myocardial infarction | 4 (1%) | 3 (1%) | 8 (1%) | 0.12 |
| Peri-procedural stroke | 11 (2%) | 3 (1%) | 9 (1%) | 0.38 |
| Coronary occlusion | 4 (1%) | 2 (1%) | 4 (1%) | 0.24 |
| Annulus rupture | 3 (1%) | 0 | 1 (0%) | 0.30 |
| Pacemaker implantation | 92 (15%) | 38 (18%) | 96 (16%) | 0.41 |
| Post-procedural biomarkers | ||||
| Minimum haemoglobin, g/dL | 10.2 [9.3–11.1] | 10.7 [9.7–12.0] | 10.1 [8.9–11.0] | 0.36 |
| Minimum platelets, n/dL | 105 [81–137] | 124 [96–162] | 116 [93–147] | 0.29 |
| Minimum eGFR, mL/m2 | 58 [42–84] | 52 [38–81] | 54 [37–70] | 0.34 |
| Post-procedural echocardiography | ||||
| Left ventricular ejection fraction, % | 60 [50–65] | 58 [48–65] | 60 [54–67] | 0.42 |
| Peak transvalvular pressure gradient, mmHg | 16 [12–23] | 14 [9–17] | 17 [12–23] | 0.26 |
| Mean transvalvular pressure gradient, mmHg | 7 [3–11] | 8 [6–11] | 8 [6–12] | 0.61 |
| Aortic insufficiency ≥ moderate | 85 (14%) | 18 (9%) | 82 (13%) | 0.16 |
| Hospitalization length (days) | 9 [7–15] | 8 [6–14] | 6 [5–9] | 0.07 |
Statistics presented: median [quartile 1–quartile 3], n (%). Abbreviations: ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; and eGFR, estimated glomerular filtration rate.
Table 2.
Prediction of all-cause mortality
| Receiver-operator curve analysis | |||||||
|---|---|---|---|---|---|---|---|
| Area under the curve (95% confidence intervals) | P-value | Sensitivity, % | Specificity, % | Positive predictive value, % | Negative predictive value, % | Accuracy, % | |
| Baseline characteristics | |||||||
| Age | 0.51 (0.44–0.57) | 0.83 | 49 (38–60) | 63 (59–67) | 18 (13–22) | 88 (85–92) | 61 (60–62) |
| Weight | 0.50 (0.42–0.58) | 0.98 | 30 (19–41) | 73 (69–77) | 16 (10–23) | 82 (77–86) | 63 (62–64) |
| Body mass index | 0.51 (0.43–0.57) | 0.80 | 26 (16–36) | 84 (80–89) | 22 (13–31) | 85 (81–90) | 74 (73–75) |
| Bicuspid aortic valve | 0.51 (0.44–0.58) | 0.72 | 38 (29–47) | 59 (51–66) | 25 (16–35) | 64 (58–71) | 61 (52–70) |
| EuroScore II | 0.56 (0.49–0.63) | 0.08 | 47 (36–58) | 36 (32–40) | 11 (8–14) | 80 (75–86) | 37 (36–38) |
| Comorbidities and past medical history | |||||||
| Diabetes | 0.57 (0.49–0.65) | 0.09 | 51 (40–61) | 64 (60–68) | 11 (6–15) | 84 (81–87) | 62 (61–63) |
| Atrial fibrillation | 0.54 (0.47–0.61) | 0.24 | 26 (17–36) | 64 (60–68) | 11 (6–15) | 84 (80–88) | 59 (58–60) |
| History of PCI | 0.53 (0.46–0.61) | 0.37 | 29 (20–38) | 67 (62–73) | 17 (11–23) | 76 (70–83) | 61 (60–62) |
| History of valve surgery | 0.52 (0.45–0.59) | 0.64 | 25 (18–32) | 63 (60–67) | 18 (11–26) | 82 (77–87) | 59 (58–60) |
| History of pacemaker implantation | 0.51 (0.43–0.58) | 0.88 | 17 (9–25) | 76 (72–81) | 16 (9–24) | 77 (73–79) | 67 (66–68) |
| History of a cerebrovascular accident | 0.53 (0.46–0.61) | 0.42 | 20 (15–25) | 79 (72–86) | 24 (19–30) | 84 (80–88) | 64 (63–65) |
| Baseline biomarkers | |||||||
| Creatinine | 0.62 (0.56–0.69) | <0.001 | 72 (62–82) | 56 (51–62) | 22 (17–27) | 92 (89–95) | 58 (57–59) |
| eGFR | 0.61 (0.55–0.68) | 0.001 | 57 (47–68) | 24 (20–27) | 11 (8–14) | 77 (71–84) | 28 (27–29) |
| Haemoglobin | 0.54 (0.48–0.60) | 0.22 | 38 (27–49) | 50 (46–55) | 11 (7–14) | 83 (79–88) | 48 (47–49) |
| Platelets | 0.57 (0.50–0.63) | 0.05 | 63 (53–74) | 53 (48–57) | 19 (14–23) | 89 (86–93) | 54 (53–55) |
| Baseline echocardiography | |||||||
| Left ventricular ejection fraction | 0.56 (0.49–0.62) | 0.11 | 76 (67–85) | 11 (8–13) | 12 (9–15) | 73 (62–83) | 20 (19–21) |
| Effective orifice area | 0.51 (0.43–0.59) | 0.74 | 18 (9–26) | 85 (82–89) | 17 (8–25) | 86 (83–89) | 75 (74–76) |
| Peak transvalvular pressure gradient | 0.53 (0.45–0.61) | 0.40 | 11 (4–18) | 83 (79–86) | 10 (4–16) | 85 (82–88) | 73 (72–74) |
| Mean transvalvular pressure gradient | 0.52 (0.44–0.60) | 0.65 | 10 (3–17) | 83 (80–87) | 9 (3–15) | 85 (82–88) | 73 (72–74) |
| Procedure––valve implantation | |||||||
| Contrast media volume | 0.55 (0.48–0.63) | 0.17 | 64 (54–74) | 42 (38–46) | 15 (11–19) | 88 (84–92) | 45 (44–46) |
| Fluoroscopy time | 0.55 (0.47–0.62) | 0.22 | 48 (37–59) | 63 (59–67) | 17 (12–22) | 88 (85–92) | 61 (60–62) |
| Radiation dose | 0.56 (0.48–0.64) | 0.10 | 24 (18–30) | 86 (83–89) | 22 (16–29) | 78 (72–85) | 72 (71–73) |
| Peri-procedural and in-hospital outcomes | |||||||
| Major vascular complication | 0.55 (0.48–0.62) | 0.13 | 13 (6–20) | 94 (91–98) | 37 (19–54) | 86 (83–90) | 81 (80–82) |
| Minor vascular complication | 0.52 (0.45–0.59) | 0.56 | 23 (17–29) | 79 (73–86) | 28 (24–33) | 75 (70–81) | 71 (70–72) |
| Life threatening bleeding | 0.57 (0.49–0.64) | 0.06 | 16 (8–24) | 93 (92–95) | 54 (34–74) | 87 (85–90) | 80 (79–81) |
| Total RBC concentrate transfused | 0.62 (0.55–0.70) | <0.001 | 23 (14–32) | 94 (92–97) | 52 (36–69) | 88 (85–91) | 81 (78–85) |
| Post-procedural biomarkers | |||||||
| Minimum haemoglobin | 0.56 (0.50–0.61) | 0.16 | 89 (83–95) | 7 (4–9) | 13 (10–16) | 80 (67–92) | 18 (17–19) |
| Minimum platelets | 0.53 (0.44–0.61) | 0.53 | 88 (81–96) | 18 (14–21) | 15 (11–19) | 91 (85–98) | 28 (27–29) |
| Minimum eGFR | 0.67 (0.59–0.74) | <0.001 | 15 (7–24) | 53 (49–58) | 5 (2–7) | 81 (76–86) | 49 (48–50) |
| Post-procedural echocardiography | |||||||
| Left ventricular ejection fraction | 0.64 (0.56–0.72) | 0.01 | 37 (23–52) | 36 (31–41) | 7 (4–10) | 81 (75–88) | 36 (35–37) |
| Peak transvalvular pressure gradient | 0.57 (0.50–0.65) | 0.16 | 27 (17–37) | 82 (78–86) | 19 (11–26) | 86 (83–90) | 72 (71–73) |
| Mean transvalvular pressure gradient | 0.53 (0.44–0.61) | 0.72 | 28 (18–37) | 82 (78–87) | 21 (13–28) | 86 (83–90) | 73 (72–74) |
| Aortic insufficiency ≥ moderate | 0.54 (0.45–0.63) | 0.42 | 32 (29–36) | 61 (58–64) | 16 (12–20) | 63 (59–68) | 51 (50–52) |
| Hospitalization length | 0.62 (0.53–0.71) | 0.014 | 73 (63–83) | 33 (29–37) | 15 (11–18) | 88 (84–93) | 38 (37–39) |
Abbreviations: eGFR, estimated glomerular filtration rate; PCI, percutaneuous coronary intervention; and RBC, red blood cell.
Figure 2.
Feature importance for the machine learning model predicting all-cause mortality in TAVI recipients. The solid bars and error bars represent the mean gain and standard deviation derived from the distribution of the importance within 10 folds of the cross-testing for each variable. Abbreviations: eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PPG, peak pressure gradient; and RBC, red blood cell.
Predictive performance
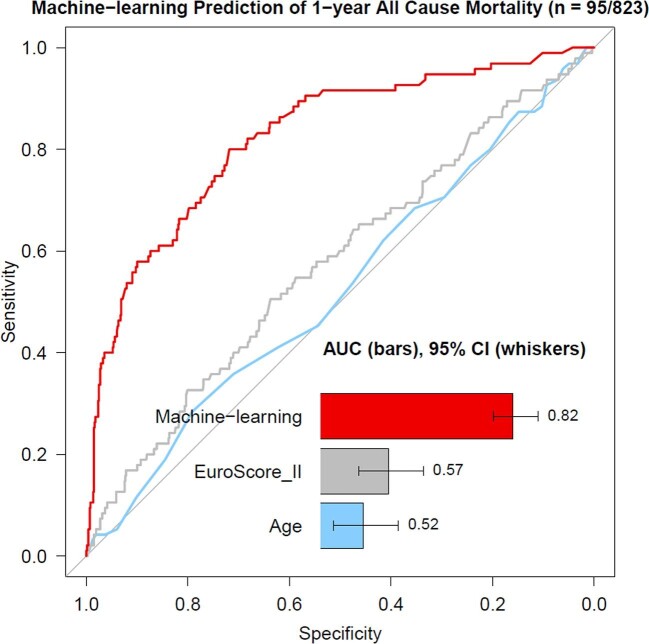
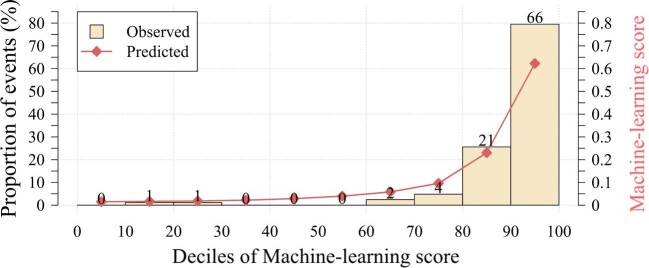
Our model was validated on a cohort of 823 consecutive patients (46% men, 82 ± 5 years old, EuroSCORE II 4.7 [2.9–6.0]%) who underwent TAVI between January 2014 and March 2019 (Table 1). Over the 12 months of follow-up, 95 (12%) subjects died in the validation cohort. Our model had an AUC of 0.82 (0.78–0.87) for prediction of 12-month mortality, which was superior to the EuroSCORE II 0.57 (0.51–0.64) and age 0.52 (0.46–0.59), P < 0.001 for a difference (Figure 3). Our model also outperformed the France II and OBSERVANT risk scores AUC of 0.58 (0.53–0.63) and 0.59 (0.54–65), respectively, P < 0.001 for a difference (Supplementary material online, Figure 1). To generate distinct clinical risk groups, we dichotomized the population according to their machine learning risk score, with the optimal cutoff for event prediction derived using the Youden index. A threshold of 15% achieved a sensitivity, specificity, and negative predictive value of 80 (72–88%), 73 (69–77%), and 96 (95–98%), respectively, for the primary endpoint. The performance of the model is also depicted on the calibration plot (Figure 4), which allows the evaluation of the agreement between machine learning scores and the actual distribution of the observed events. In the external validation, 77 (91%) events occurred in patients with the machine learning score within the top two deciles. We have also developed a machine learning model based only on pre-procedural data; however, its performance was limited—AUC 0.64 (0.59–0.69).
Figure 3.
Prediction of 1-year all-cause mortality on external testing. Receiver-operating characteristic curves for prediction of 1-year all-cause mortality following hospital discharge after successful transcatheter aortic valve implantation. The machine learning XGBoost model had a significantly higher area under the curve for all-cause mortality prediction than an established risk score (EuroSCORE II) and conventional predictors of outcomes (including age), P < 0.001.
Figure 4.
Calibration plot for machine learning XGBoost model. The calibration plot shows the relationship between the observed and predicted proportion of events, grouped by decile of risk. The XGBoost model showed good calibration with the observed 1-year risk of all-cause mortality.
Individualized explainability of the prediction
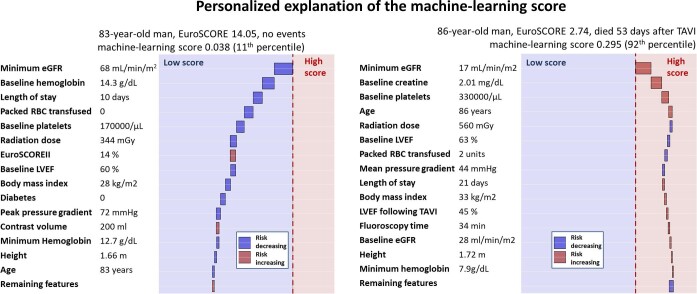
To further clarify and explain the machine learning predictions, we also provide waterfall plots that highlight the contribution of the predictors for individual patients. As demonstrated in Figure 5 and Supplementary material online, Figure 1, the machine learning model can accurately predict events in elderly patients who present with a high EuroSCORE II as well as relatively younger individuals who are at a low risk according to the surgical risk scores.
Figure 5.
Individual prediction of all-cause mortality with explainable artificial intelligence. Explanations of individual prediction with subject-specific feature importance for an 83-year-old man who survived over a year following TAVI and an 86-year-old man who died 53 days after TAVI. The x-axis corresponds to the machine learning risk score. The arrows represent the influence of each covariate on the overall prediction; blue and red arrows indicate whether the associated parameters decrease (blue) or increase (red) the risk of future events. The combination of all covariates’ influence provides the final machine learning risk score. The red and blue colours provide the separation between low and high machine learning risk scores. Abbreviations: eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; and RBC, red blood cell.
Discussion
In this multicentre machine learning study, we have demonstrated that by leveraging state-of-the-art artificial intelligence and readily available clinical data, it is feasible to predict 1-year all-cause mortality in patients who have undergone successful TAVI and were discharged from the hospital following the procedure. Our approach outperformed individual clinical metrics, as well as the EuroSCORE II risk score, which despite having been developed to predict short-term adverse events following surgical valve replacement and because of the lack of bespoke risk stratification tools for TAVI, is frequently used in patients, managed percutaneously. The observed efficacy of our model suggests that machine learning could play an important clinical role in evaluating prognostic risk in patients after TAVI. By stratifying TAVI recipients according to the risk of adverse events, it could facilitate offering a more intense follow-up regimen to those at the highest risk of adverse outcomes after the index procedure. Ultimately, it can enable cost-effective allocation of medical resources and might contribute to improving outcomes following TAVI.
In view of the complexity of patients undergoing TAVI, artificial intelligence with machine learning emerges as an ideal tool for combining the information provided by a large set of weak predictors for robust risk stratification. The XGBoost algorithm has been successfully implemented for risk prediction in a wide range of clinical scenarios.14,15 It enables the incorporation of numerous predictors into the model even when these variables are correlated—a major limitation with conventional regression analyses. Our model was developed using conservative internal 10-fold repeated validation, which limits overfitting and ascertains generalizability. Importantly, the model attained high accuracy in external validation (combined data from two independent tertiary clinical centres) by objectively integrating pre- and peri-procedural data—a task that is challenging to accomplish at the point of care.
While surgical risk scores have been widely used to stratify different groups of patients for comparative clinical trials between SAVR and TAVI, they were developed and validated on series of patients undergoing SAVR and therefore do not necessarily encompass the diverse co-morbidities that have an adverse impact on outcomes in TAVI recipients. Only a few studies have aimed to develop methods for risk stratification beyond 30 days after TAVI and less than a handful included peri-procedural data in the models.8–12 Moreover, their performance was limited, or the target population was only extreme-risk and high-risk patients.22–26 Our model addresses this important clinical gap. By providing patient-specific risk estimates, it has the potential to enable tailored post-discharge patient surveillance after TAVI, ultimately providing an opportunity for a more cost-effective allocation of resources.
Prediction of TAVI outcomes is challenging due to the competing cardiovascular and non-cardiovascular risks, as well as the fact that patients are typically fairly homogeneous in terms of conventional metrics that act as robust predictors in different clinical settings (i.e. age).27 While these are not adequately accounted for by the EuroSCORE II, machine learning has the potential to overcome this issue and risk-stratify the challenging cohort of TAVI patients. In our study, none of the single metrics acted as a strong predictor of all-cause mortality on receiver-operator curve characteristic analysis (AUC < 0.70, Table 2). As shown previously, the lack of association between age and survival in our study population may be caused by a ceiling effect in the overall intermediate-risk cohort.27
Importantly, the machine learning model also provides insights into the top predictors by ranking the relative contribution from each variable for a unique patient. This has the potential to improve physicians’ confidence in the machine learning results and may potentially help to overcome the perception of artificial intelligence as a ‘black box’.28 Our model represents a substantial improvement in risk stratification. While multiple variables have been shown to act as independent predictors of adverse outcomes following TAVI, prior studies did not separate data for deriving independent predictors and testing their clinical utility, and therefore the predictive value of such parameters may be overestimated and not applicable to TAVI recipients globally. In contrast, in our study we employed rigorous external validation, establishing the generalizability of our model on unseen data from two independent centres.
Limitations
Our study has notable strengths and weaknesses. Our model was trained and tested on data from tertiary TAVI centres that reflect everyday clinical practice (a real-world setting). It is based on readily available data, and therefore the proposed approach could easily benefit patients without the need for additional testing or tedious data crunching. Indeed, our machine learning approach could be easily incorporated into clinical practice. At the time of discharge from the hospital after a successful TAVI, it could inform the physician on the risk of all-cause mortality during the first year following the procedure, enabling patient-specific post-discharge care planning. While our databases lack STS-PROM scores, we have provided the EuroSCORE II, which was shown to have comparable discrimination and calibration to STS-PROM in patients receiving aortic valve replacement.29,30 In the derivation cohort, we included patients who underwent TAVI in 2013–19, ascertaining a large population for establishing our model but inevitably also including a relatively large proportion of high-risk patients who currently represent a minority of TAVI recipients. Our model did not include scores that characterize patients’ frailty, concomitant coronary artery disease, or the perception of their health status. Additionally, due to the observational nature of our study, we acknowledge the inherent risk for selection bias and residual confounding, which is, however, limited given the multicentre character of the current study. Finally, while prediction of outcomes following hospital discharge is valuable, our study does not address the need for a pre-procedural tool for prediction of adverse outcomes that could facilitate selection of patients for TAVI. Given the limited performance of models based exclusively on pre-procedural clinical data, it appears that inclusion of advanced cardiac imaging data might be necessary.31,32 Ultimately, robust models based on clinical and imaging pre-procedural data could have a significant impact on clinical practice.
Conclusions
In conclusion, machine learning based on readily available clinical data allows accurate prediction of 1-year all-cause mortality following TAVI. The machine learning model could potentially be used to guide the intensity of patients’ follow-up and post-discharge care after TAVI.
Supplementary Material
Contributor Information
Jacek Kwiecinski, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland; Departments of Medicine (Division of Artificial Intelligence in Medicine) and Biomedical Sciences, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Metro 203, Los Angeles, CA 90048, USA.
Maciej Dabrowski, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Luis Nombela-Franco, Cardiovascular Institute, Hospital Clinico San Carlos, IdISSC, Madrid, Spain.
Kajetan Grodecki, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Konrad Pieszko, Departments of Medicine (Division of Artificial Intelligence in Medicine) and Biomedical Sciences, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Metro 203, Los Angeles, CA 90048, USA; Department of Interventional Cardiology and Cardiac Surgery, University of Zielona Gora, Zielona Gora, Poland.
Zbigniew Chmielak, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Anna Pylko, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Breda Hennessey, Cardiovascular Institute, Hospital Clinico San Carlos, IdISSC, Madrid, Spain.
Lukasz Kalinczuk, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Gabriela Tirado-Conte, Cardiovascular Institute, Hospital Clinico San Carlos, IdISSC, Madrid, Spain.
Bartosz Rymuza, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Janusz Kochman, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Maksymilian P Opolski, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Zenon Huczek, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Marc R Dweck, Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, UK.
Damini Dey, Departments of Medicine (Division of Artificial Intelligence in Medicine) and Biomedical Sciences, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Metro 203, Los Angeles, CA 90048, USA.
Pilar Jimenez-Quevedo, Cardiovascular Institute, Hospital Clinico San Carlos, IdISSC, Madrid, Spain.
Piotr Slomka, Departments of Medicine (Division of Artificial Intelligence in Medicine) and Biomedical Sciences, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Metro 203, Los Angeles, CA 90048, USA.
Adam Witkowski, Department of Interventional Cardiology and Angiology, Institute of Cardiology, Warsaw, Poland.
Funding
This research was funded by the National Science Centre, Poland (grant 2021/41/B/NZ5/02630). K.G. is supported by the Foundation for Polish Science. M.R.D. is a recipient of the Sir Jules Thorn Award for Biomedical Research Award (2015) and is supported by the British Heart Foundation (FS/14/78/31020). This research was supported in part by grants R01HL135557, R01HL148787, and R01HL151266 from the National Heart, Lung, and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest:
The authors declare that they have no relevant conflict of interest or material/financial interests that relate to the research described in this paper.
Data availability
The data underlying this article were provided by the Institute of Cardiology, Warsaw, the Medical University of Warsaw, and the Cardiovascular Institute, Hospital Clinico San Carlos, Madrid. Data can be shared on request to the corresponding author with permission from the Institute of Cardiology, the Medical University of Warsaw, the Cardiovascular Institute, Hospital Clinico San Carlos, and the Polish/Spanish Data Protection Agency.
References
- 1. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer Fet al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo Met al. Transcatheter aortic valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair Det al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 4. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJet al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 201973;:546–553. [DOI] [PubMed] [Google Scholar]
- 5. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel Get al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492–2516. [DOI] [PubMed] [Google Scholar]
- 6. Werner N, Zahn R, Beckmann A, Bauer T, Bleiziffer S, Hamm CWet al. Patients at intermediate surgical risk undergoing isolated interventional or surgical aortic valve implantation for severe symptomatic aortic valve stenosis. Circulation 2018;138:2611–2623. [DOI] [PubMed] [Google Scholar]
- 7. Bekeredjian R, Szabo G, Balaban Ü, Bleiziffer S, Bauer T, Ensminger Set al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: in-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur Heart J 2019;40:1323–1330. [DOI] [PubMed] [Google Scholar]
- 8. Iung B, Laouénan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge Pet al. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart 2014;100:1016–1023. [DOI] [PubMed] [Google Scholar]
- 9. Capodanno D, Barbanti M, Tamburino C, D'Errigo P, Ranucci M, Santoro Get al. A simple risk tool (the OBSERVANT score) for prediction of 30-day mortality after TAVI. Am J Cardiol 2014;113:1851–1858. [DOI] [PubMed] [Google Scholar]
- 10. Edwards FH, Cohen DJ, O′Brien SM, Peterson ED, Mack MJ, Shahian DMet al. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 11. Martin GP, Sperrin M, Ludman PF, de Belder MA, Redwood SR, Townend JNet al. Novel United Kingdom prognostic model for 30-day mortality following transcatheter aortic valve implantation. Heart 2018;104:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez-Suarez DF, Kim Y, Villablanca P, Gupta T, Wiley J, Nieves-Rodriguez BGet al. A machine learning prediction models for in-hospital mortality after transcatheter aortic valve replacement. JACC: Cardiovasc Interv 2019;12:1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PPet al. Artificial intelligence in cardiovascular imaging J Am Coll Cardiol 2019;73: 1317–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Commandeur F, Slomka PJ, Goeller M, Chen X, Cadet S, Razipour Aet al. Machine learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res 2020;116:2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwiecinski J, Tzolos E, Meah MN, Cadet S, Adamson PD, Grodecki Ket al. Machine learning with 18F-sodium fluoride PET and quantitative plaque analysis on CT angiography for the future risk of myocardial infarction. J Nucl Med 2022;63:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (KDD '16). New York, NY:Association for Computing Machinery, pp. 785–794. 10.1145/2939672.2939785. [DOI] [Google Scholar]
- 17. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JPet al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 18. Kim JH. Estimating classification error rate: repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal 2009;53:3735–3745. [Google Scholar]
- 19. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning Data Mining, Inference and Prediction. New York: Springer. 2001; 367. [Google Scholar]
- 20. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ’16, August 13-17, 2016, San Francisco, CA. pp. 785–794. [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 22. Agasthi P, Ashraf H, Pujari SH, Girardo ME, Tseng A, Mookadam Fet al. Artificial intelligence trumps TAVI2-SCORE and CoreValve score in predicting 1-year mortality post-transcatheter aortic valve replacement. Cardiovasc Revasc Med 2021;24:33–41. [DOI] [PubMed] [Google Scholar]
- 23. Lantelme P, Eltchaninoff H, Rabilloud M, Souteyrand G, Dupré M, Spaziano Met al. Development of a risk score based on aortic calcification to predict 1-year mortality after transcatheter aortic valve replacement. JACC: Cardiovasc Imaging 2019;12:123–132. [DOI] [PubMed] [Google Scholar]
- 24. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo Jet al. Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:343–352. [DOI] [PubMed] [Google Scholar]
- 25. Lopes RR, van Mourik MS, Schaft EV, Ramos LA, Baan J Jr, Vendrik Jet al. Value of machine learning in predicting TAVI outcomes. Neth Heart J 2019;27:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penso M, Pepi M, Fusini L, Muratori M, Cefalù C, Mantegazza Vet al. Predicting long-term mortality in TAVI patients using machine learning techniques. J Cardiovasc Dev Dis 2021;8:44. doi: 10.3390/jcdd8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinsson A, Nielsen SJ, Milojevic M, Redfors B, Omerovic E, Tønnessen Tet al. Life expectancy after surgical aortic valve replacement. J Am Coll Cardiol 2021;78:2147–2157. [DOI] [PubMed] [Google Scholar]
- 28. Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell 2019;1: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang TK, Choi DH, Stewart R, Gamble G, Haydock D, Ruygrok Pet al. Comparison of four contemporary risk models at predicting mortality after aortic valve replacement. J Thorac Cardiovasc Surg 2015;149:443–448. [DOI] [PubMed] [Google Scholar]
- 30. Wendt D, Thielmann M, Kahlert P, Kastner S, Price V, Al-Rashid Fet al. Comparison between different risk scoring algorithms on isolated conventional or transcatheter aortic valve replacement. Ann Thorac Surg 2014;97:796–802. [DOI] [PubMed] [Google Scholar]
- 31. Kwiecinski J, Chin CWL, Everett RJ, White AC, Semple S, Yeung Eet al. Adverse prognosis associated with asymmetric myocardial thickening in aortic stenosis. Eur Heart J Cardiovasc Imaging 2018;19:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwiecinski J, Tzolos E, Cartlidge TRG, Fletcher A, Doris MK, Bing Ret al. Native aortic valve disease progression and bioprosthetic valve degeneration in patients with transcatheter aortic valve implantation. Circulation 2021;144:1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Institute of Cardiology, Warsaw, the Medical University of Warsaw, and the Cardiovascular Institute, Hospital Clinico San Carlos, Madrid. Data can be shared on request to the corresponding author with permission from the Institute of Cardiology, the Medical University of Warsaw, the Cardiovascular Institute, Hospital Clinico San Carlos, and the Polish/Spanish Data Protection Agency.