Abstract
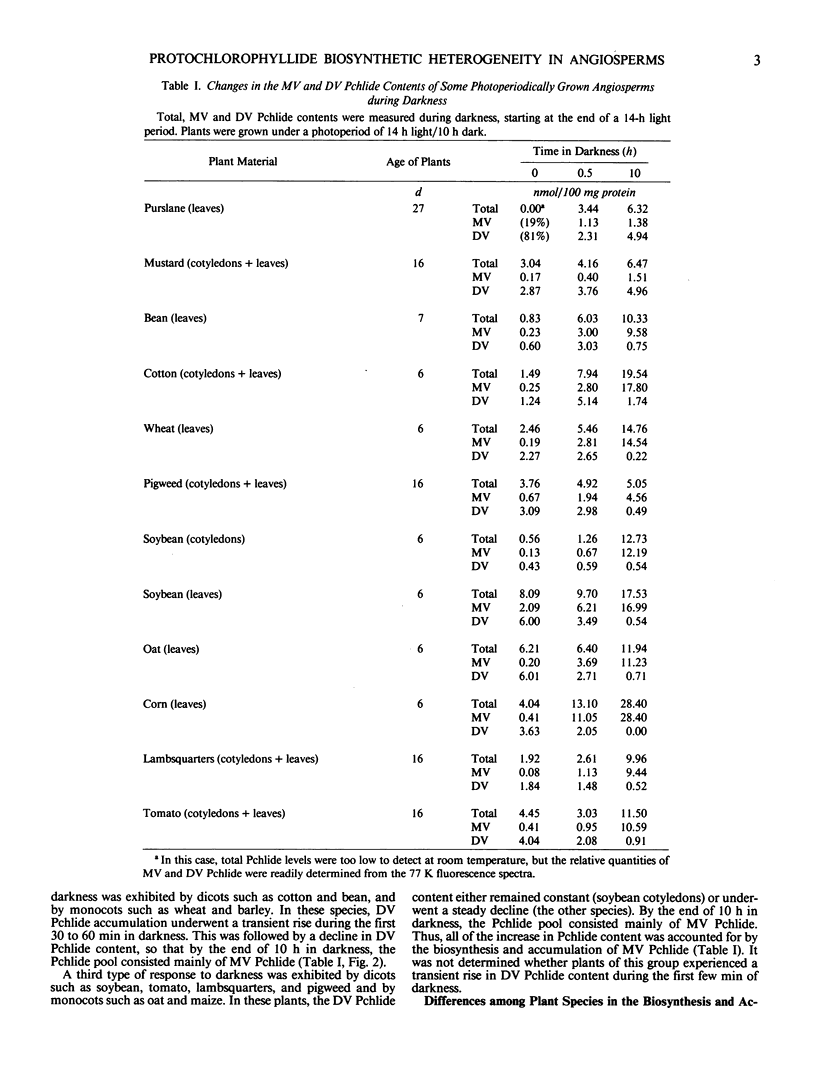
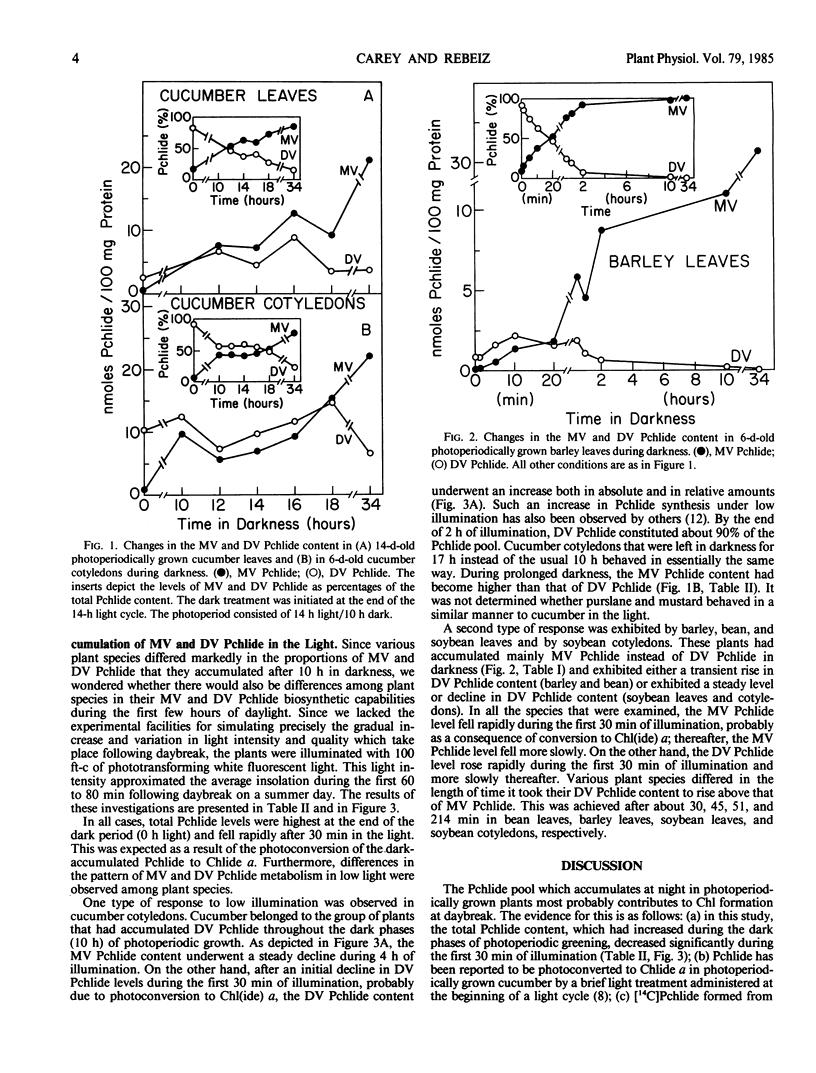
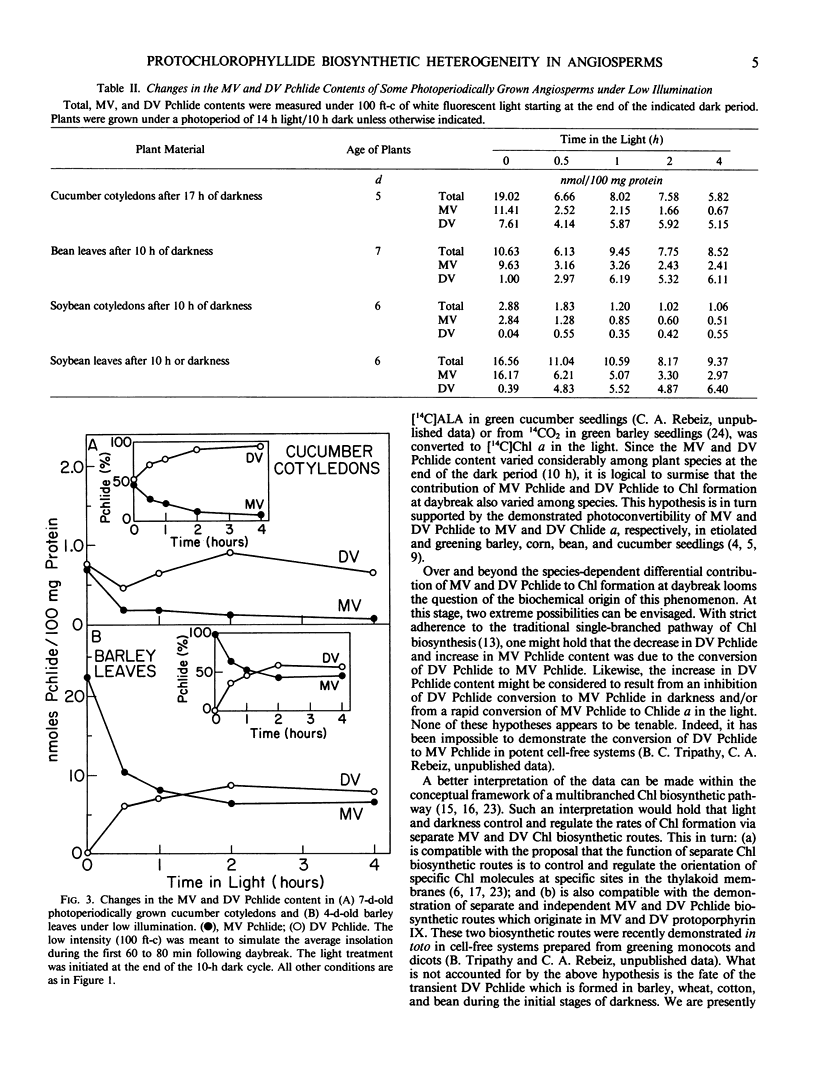
Various angiosperms differed in their monovinyl and divinyl protochlorophyllide biosynthetic capabilities during the dark and light phases of photoperiodic growth. Some plant species such as Cucumis sativus L., Brassica juncea (L.) Coss., Brassica kaber (DC.) Wheeler, and Portulaca oleracea L. accumulated mainly divinyl protochlorophyllide at night. Monocotyledonous species such as Avena sativa L., Hordeum vulgare L., Triticum secale L., Zea mays L., and some dicotyledonous species such as Phaseolus vulgaris L., Glycine max (L.) Merr., Chenopodium album L., and Lycopersicon esculentum L. accumulated mainly monovinyl protochlorophyllide at night.
Under low light intensities meant to simulate the first 60 to 80 minutes following daybreak divinyl protochlorophyllide appeared to contribute much more to chlorophyll formation than monovinyl protochlorophyllide in species such as Cucumis sativus L. Under the same light conditions, species which accumulated mainly monovinyl protochlorophyllide at night appeared to form chlorophyll preferably via monovinyl protochlorophyllide.
These results were interpreted in terms of: (a) a differential contribution of monovinyl and divinyl protochlorophyllide to chlorophyll formation at daybreak in various plant species; and (b) a differential regulation of the monovinyl and divinyl protochlorophyllide biosynthetic routes by light and darkness.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belanger F. C., Duggan J. X., Rebeiz C. A. Chloroplast biogenesis. Identification of chlorophyllide a (E458f674) as a divinyl chlorophyllide a. J Biol Chem. 1982 May 10;257(9):4849–4858. [PubMed] [Google Scholar]
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis, XXVII. Detection of novel chlorophyll and chlorophyll precursors in higher plants. Biochem Biophys Res Commun. 1979 May 28;88(2):365–371. doi: 10.1016/0006-291x(79)92057-6. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis. Detection of divinyl protochlorophyllide in higher plants. J Biol Chem. 1980 Feb 25;255(4):1266–1272. [PubMed] [Google Scholar]
- Cohen C. E., Bazzaz M. B., Fullett S. H., Rebeiz C. A. Chloroplast Biogenesis: XX. Accumulation of Porphyrin and Phorbin Pigments in Cucumber Cotyledons during Photoperiodic Greening. Plant Physiol. 1977 Nov;60(5):743–746. doi: 10.1104/pp.60.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. E., Rebeiz C. A. Chloroplast Biogenesis: XXII. Contribution of Short Wavelength and Long Wavelength Protochlorophyll Species to the Greening of Higher Plants. Plant Physiol. 1978 May;61(5):824–829. doi: 10.1104/pp.61.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto C. M., Castelfranco P. A. Separation of monovinyl and divinyl protochlorophyllides and chlorophyllides from etiolated and phototransformed cucumber cotyledons. Plant Physiol. 1983 Sep;73(1):79–81. doi: 10.1104/pp.73.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K. W., Boynton J. E. Macromolecular physiology of plastids. 8. Pigment and membrane formation in plastids of barley greening under low light intensity. J Cell Biol. 1970 Feb;44(2):290–304. doi: 10.1083/jcb.44.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., FRENCH C. S., SMITH J. H. C. The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys. 1951 Mar;31(1):1–17. doi: 10.1016/0003-9861(51)90178-6. [DOI] [PubMed] [Google Scholar]
- REBEIZ C. A., CASTELFRANCO P., ENGELBRECHT A. H. FRACTIONATION AND PROPERTIES OF AN EXTRA-MITOCHONDRIAL ENZYME SYSTEM FROM PEANUTS CATALYZING THE BETA-OXIDATION OF PALMITIC ACID. Plant Physiol. 1965 Mar;40:281–286. doi: 10.1104/pp.40.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Dec;171(2):549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Wu S. M., Kuhadja M., Daniell H., Perkins E. J. Chlorophyll a biosynthetic routes and chlorophyll a chemical heterogeneity in plants. Mol Cell Biochem. 1983;57(2):97–125. doi: 10.1007/BF00849189. [DOI] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]


