Abstract
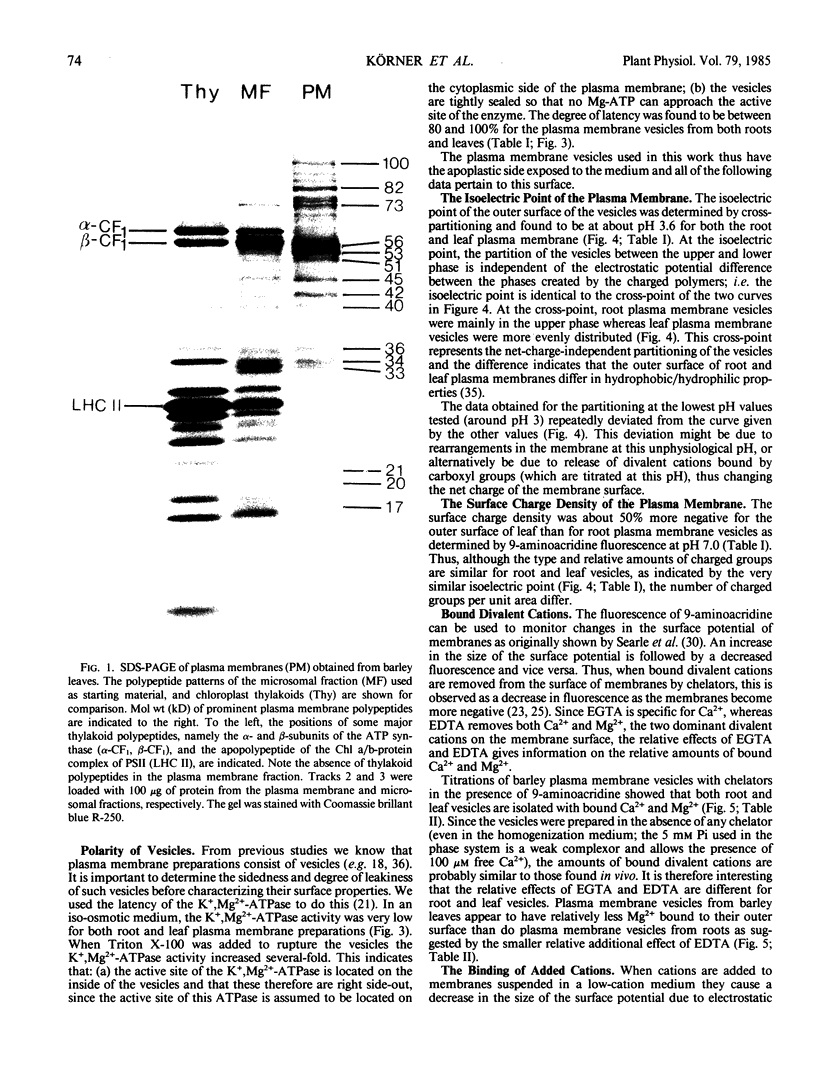
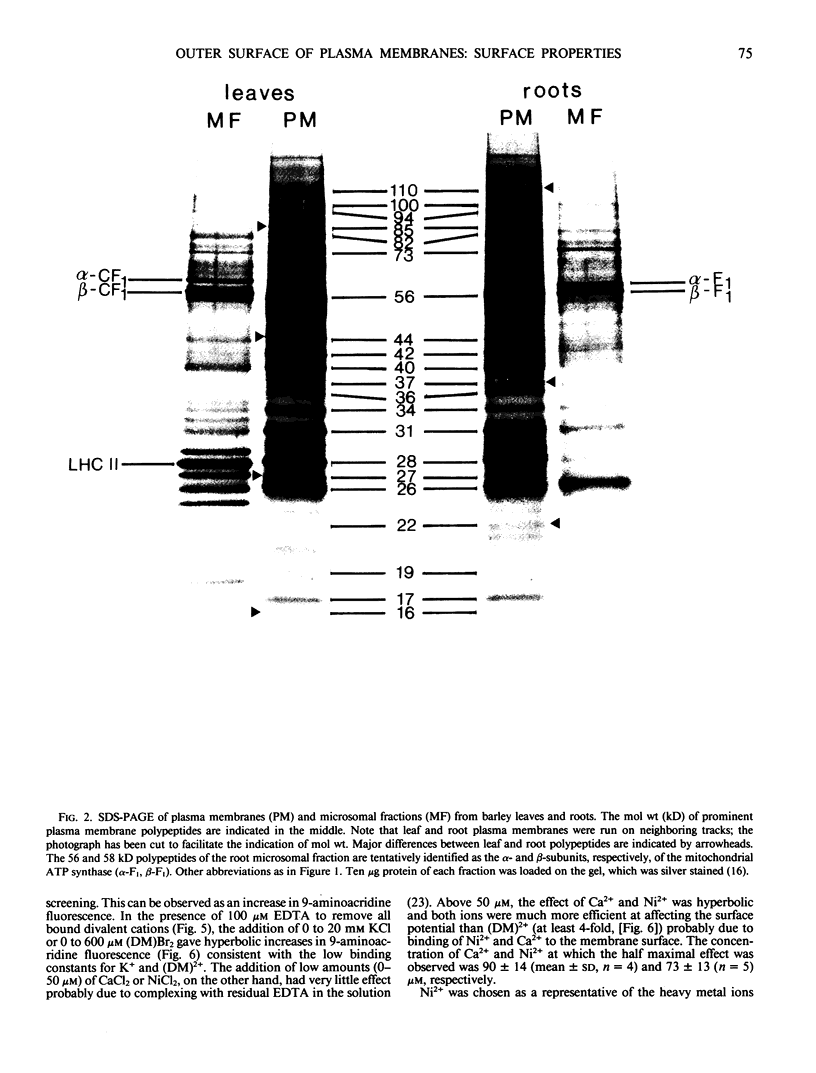
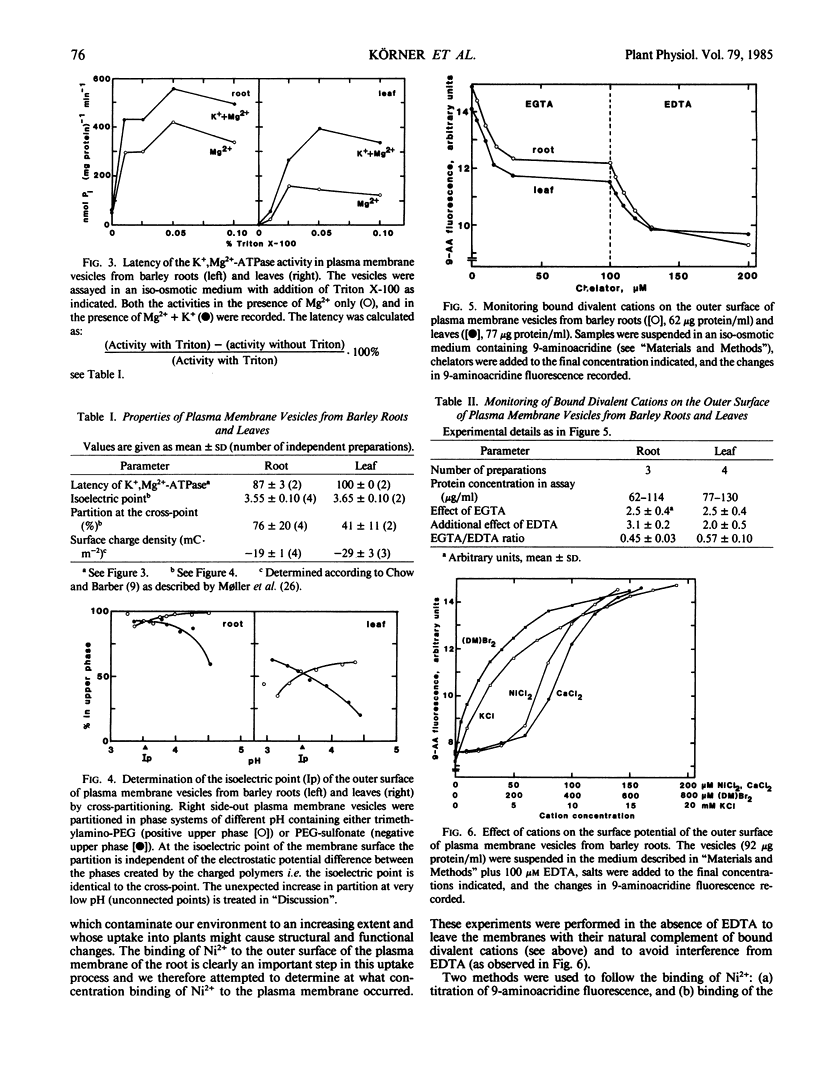
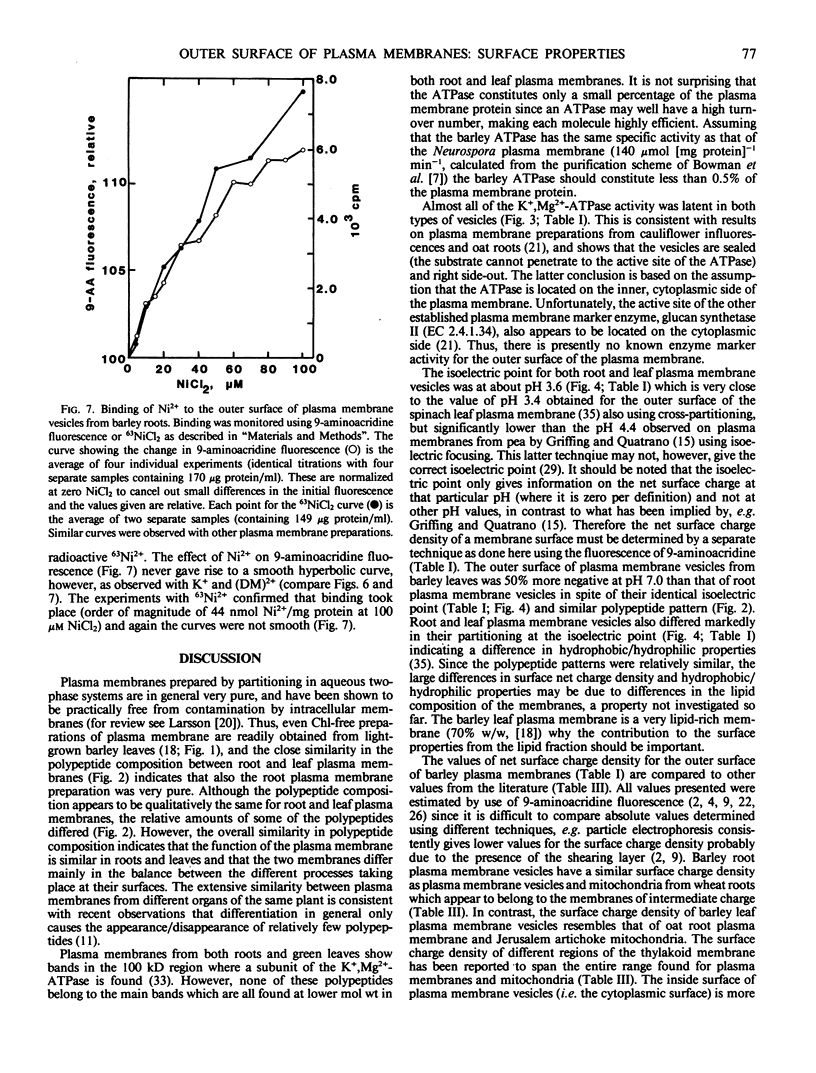
Highly purified plasma membrane vesicles were obtained from roots and leaves of 7-day-old light-grown barley (Hordeum vulgare L. cv Kristina) seedlings by partitioning of crude microsomal fractions in a dextran-polyethylene glycol two-phase system. Sodium dodecylsulfate polyacrylamide gel electrophoresis showed the polypeptide composition of plasma membranes from the two organs to be qualitatively similar, but with different relative amounts of some of the polypeptides. Between 80 and 100% of the K+,Mg2+-ATPase activity was latent indicating that the vesicles were sealed and right side-out. The isoelectric points of the outer surface of root and leaf plasma membranes as determined by cross-partitioning were similar and quite acidic—about pH 3.6. In contrast, the net negative surface charge density at pH 7.0 as measured by 9-aminoacridine fluorescence differed significantly, being −29 mC·m−2 for the leaf plasma membrane and only −19 mC·m−2 for the root plasma membrane. As isolated, both types of plasma membrane vesicles had Ca2+ and Mg2+ bound to the outer surface as shown by the combined use of chelators and 9-aminoacridine fluorescence; however, the leaf plasma membrane had a relatively higher proportion of Ca2+ bound (0.57) than did the root plasma membrane (0.45). This difference probably reflects differences in the in vivo conditions as no chelator was present during the isolation procedure. Also Ni2+ could bind to the root vesicles as indicated by the effect of Ni2+ on 9-aminoacridine fluorescence, and by the binding of 63Ni2+ (44 nanomoles bound per milligram protein) at 100 micromolar NiCl2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund H. E., Andersson B., Persson A., Albertsson P. A. Isoelectric points of spinach thylakoid membrane surfaces as determined by cross partition. Biochim Biophys Acta. 1979 Apr 4;552(2):238–246. doi: 10.1016/0005-2736(79)90280-3. [DOI] [PubMed] [Google Scholar]
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Nickel in plants: I. Uptake kinetics using intact soybean seedlings. Plant Physiol. 1978 Oct;62(4):563–565. doi: 10.1104/pp.62.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow W. S., Barber J. Salt-dependent changes of 9-aminoacridine fluorescence as a measure of charge densities of membrane surfaces. J Biochem Biophys Methods. 1980 Sep;3(3):173–185. doi: 10.1016/0165-022x(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Colleen S., Hovelius B., Wieslander A., Mårdh P. A. Surface properties of Staphylococcus saprophyticus and Staphylococcus epidermidis as studied by adherence tests and two-polymer, aqueous phase systems. Acta Pathol Microbiol Scand B. 1979 Dec;87(6):321–328. doi: 10.1111/j.1699-0463.1979.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Ericson I. Determination of the isoelectric point of rat liver mitochondria by cross-partition. Biochim Biophys Acta. 1974 Jul 12;356(1):100–107. doi: 10.1016/0005-2736(74)90297-1. [DOI] [PubMed] [Google Scholar]
- Griffing L. R., Quatrano R. S. Isoelectric focusing of plant cell membranes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4804–4808. doi: 10.1073/pnas.81.15.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Møller I. M., Johnston S. P., Palmer J. M. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981 Feb 15;194(2):487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Kay C. J., Palmer J. M. Electrostatic screening stimulates rate-limiting steps in mitochondrial electron transport. Biochem J. 1984 Nov 1;223(3):761–767. doi: 10.1042/bj2230761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G. F., Barber J., Mills J. D. 9-amino-acridine as a probe of the electrical double layer associated with the chloroplast thylakoid membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):413–425. doi: 10.1016/0005-2728(77)90230-4. [DOI] [PubMed] [Google Scholar]
- Telfer A., Barber J., Jagendorf A. T. Electrostatic control of chloroplast coupling factor binding to thylakoid membranes as indicated by cation effects of electron transport and reconstitution of photophosphorylation. Biochim Biophys Acta. 1980 Jul 8;591(2):331–345. doi: 10.1016/0005-2728(80)90164-4. [DOI] [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Weinstein J. N., Blumenthal R., van Renswoude J., Kempf C., Klausner R. D. Charge clusters and the orientation of membrane proteins. J Membr Biol. 1982;66(3):203–212. doi: 10.1007/BF01868495. [DOI] [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Nałecz M. J. Surface change of biological membranes as a possible regulator of membrane-bound enzymes. Eur J Biochem. 1979 Feb 15;94(1):99–107. doi: 10.1111/j.1432-1033.1979.tb12876.x. [DOI] [PubMed] [Google Scholar]