Abstract
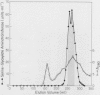
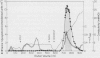
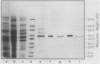
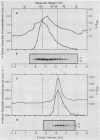
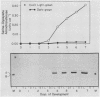
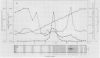
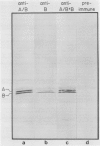
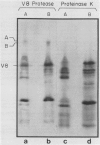
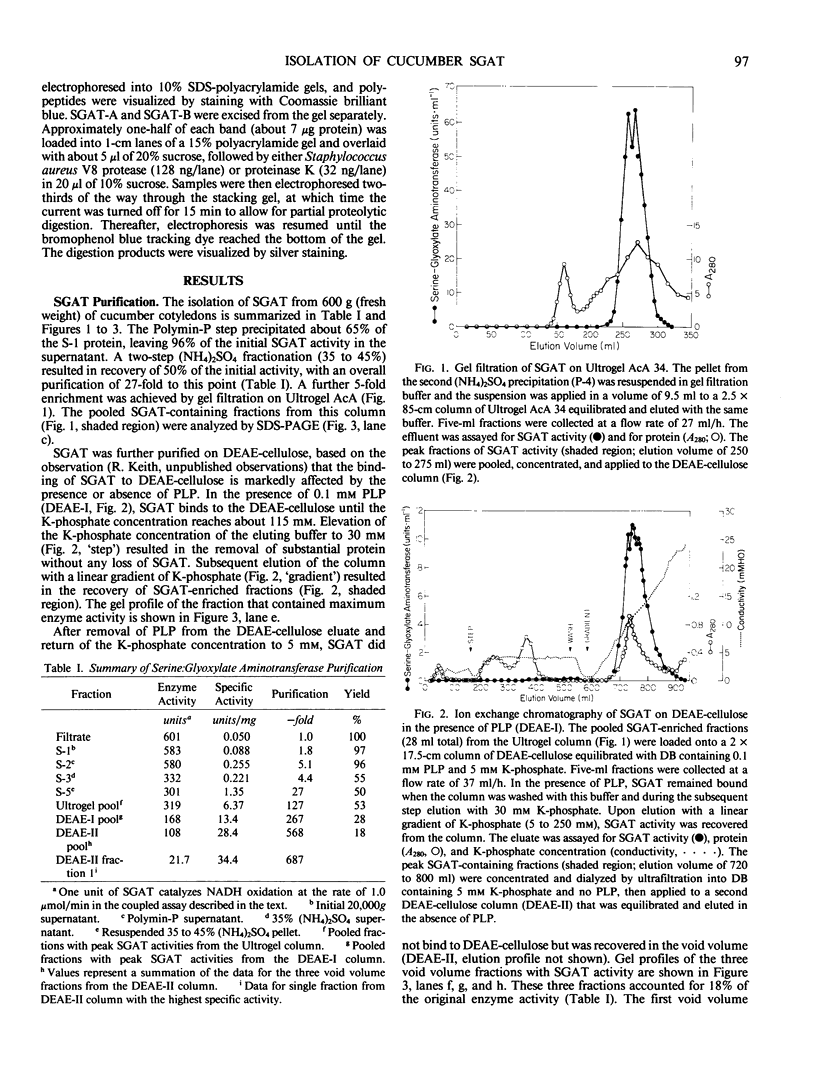
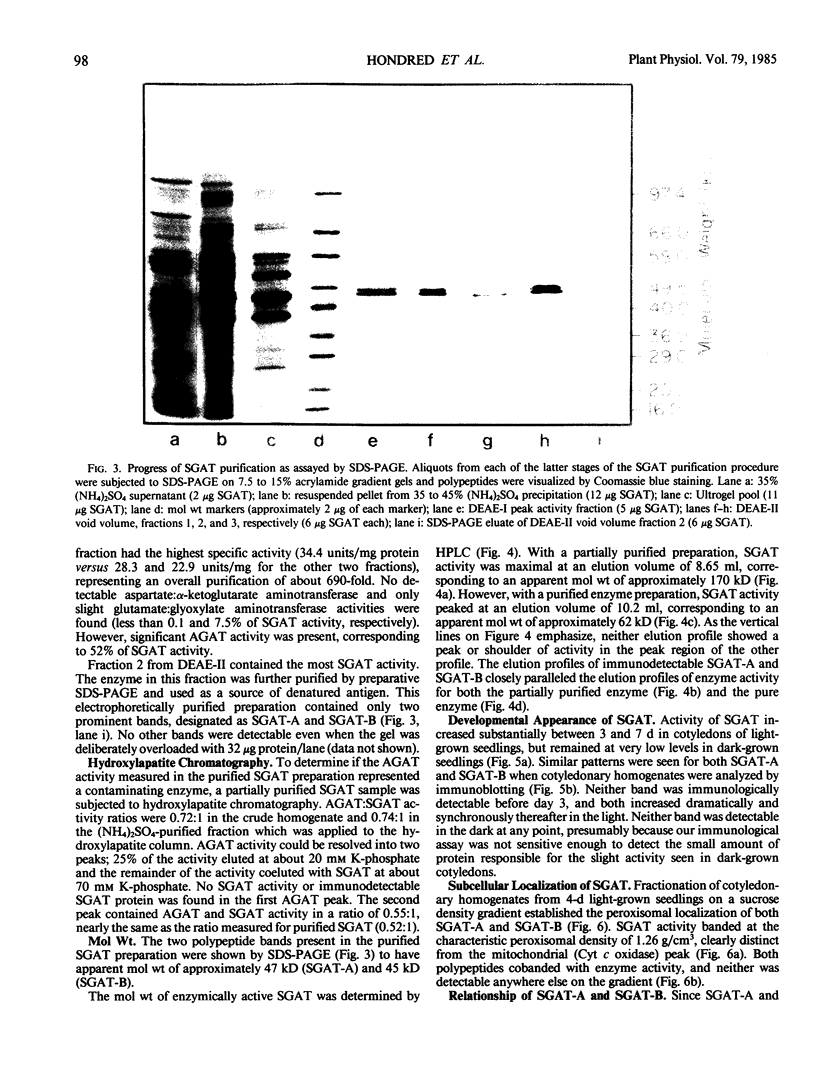
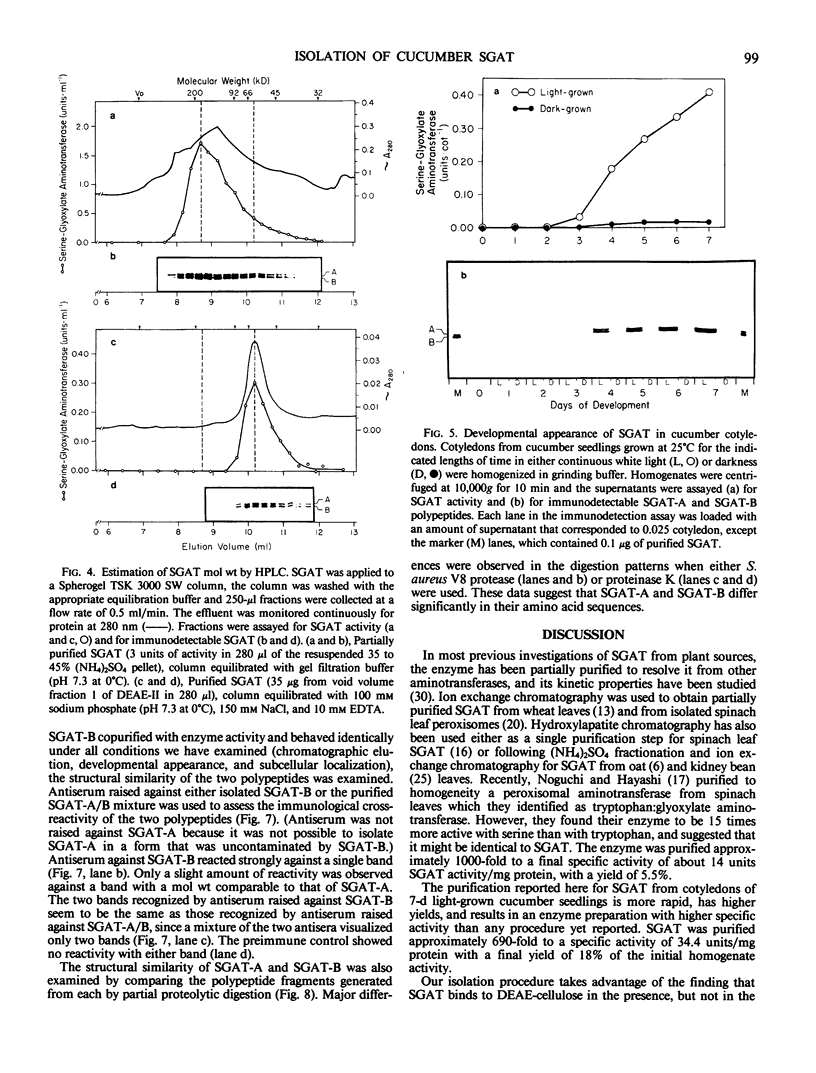
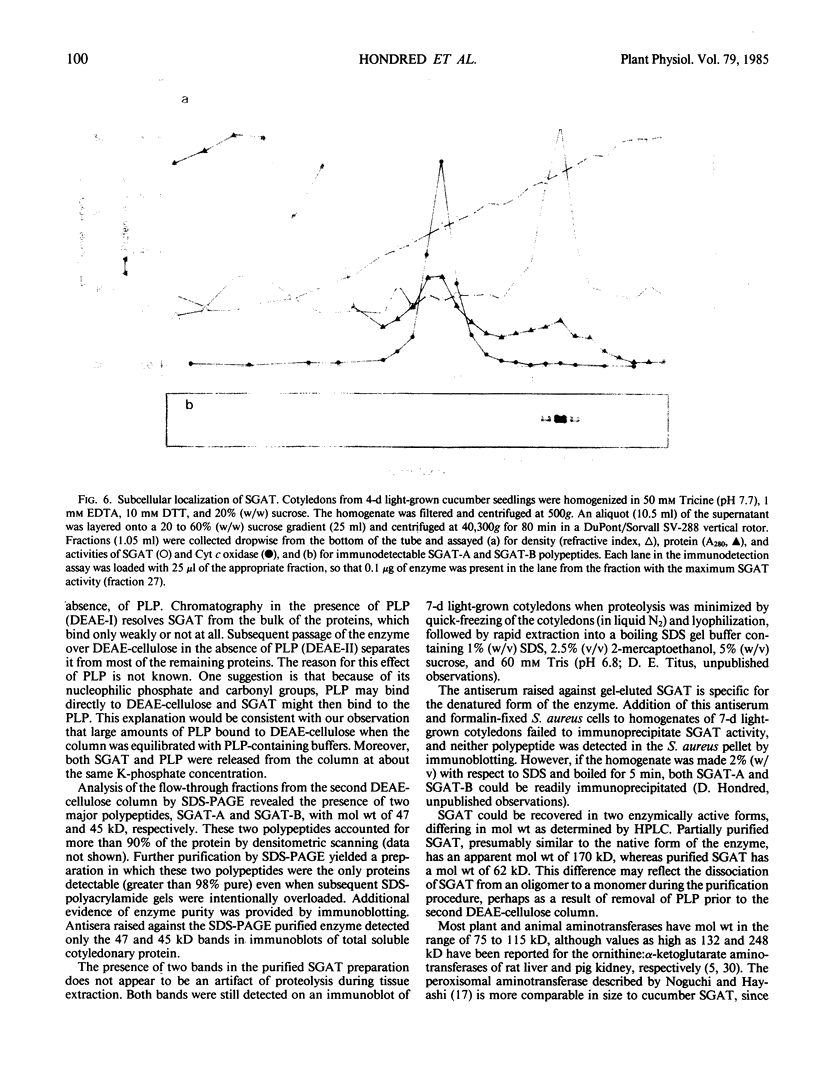
Serine:glyoxylate aminotransferase, a marker enzyme for leaf peroxisomes, has been purified to homogeneity from cucumber cotyledons (Cucumis sativus cv Improved Long Green). The isolation procedure involved precipitation with polyethyleneimine, a two-step ammonium sulfate fractionation (35 to 45%), gel filtration on Ultrogel AcA 34, and ion exchange chromatography on diethylaminoethyl-cellulose, first in the presence of pyridoxal-5-phosphate, and then in its absence. The enzyme was purified approximately 690-fold to a final specific activity of 34.4 units per milligram. Electrophoresis of the purified enzyme on sodium dodecyl sulfate-polyacrylamide gels revealed two polypeptide bands with apparent molecular weights of approximately 47,000 and 45,000. Both polypeptides coeluted with enzyme activity under all chromatographic conditions investigated, both were localized to the peroxisome, and both accumulated in cotyledons as enzyme activity increased during development. The two polypeptides appear not to be structurally related, since they showed little immunological cross-reactivity and gave rise to different peptide fragments when subjected to partial proteolytic digestion. Antiserum raised against either the denatured enzyme or the 45,000-dalton polypeptide did not react with any other polypeptides present in a crude cotyledonary homogenate. The purified enzyme also had alanine:glyoxylate aminotransferase activity, but was about twice as active with serine as the amino donor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. M., Leaver C. J., Weir E. M., Riezman H. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: I. Developmental Changes in Cotyledonary Protein, RNA, and Enzyme Activities during Germination. Plant Physiol. 1978 Oct;62(4):542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Betsche T., Gerhardt B. Apparent Catalase Synthesis in Sunflower Cotyledons during the Change in Microbody Function: A Mathematical Approach for the Quantitative Evaluation of Density-labeling Data. Plant Physiol. 1978 Oct;62(4):590–597. doi: 10.1104/pp.62.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock B. L., Wilkinson D. A., King J. Glyoxylate aminotansferases from oat leaves. Can J Biochem. 1970 Apr;48(4):486–492. doi: 10.1139/o70-078. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Trelease R. N. Cytochemical demonstration of malate synthase and glycolate oxidase in microbodies of cucumber cotyledons. Plant Physiol. 1975 Nov;56(5):710–717. doi: 10.1104/pp.56.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Waygood E. R. Glyoxylate aminotranferases from wheat leaves. Can J Biochem. 1968 Aug;46(8):771–779. doi: 10.1139/o68-118. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Silver staining methods for polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:230–239. doi: 10.1016/s0076-6879(83)96021-4. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tolbert N. E. Serine: glyoxylate, alanine:glyoxylate, and glutamate:glyoxylate aminotransferase reactions in peroxisomes from spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7631–7638. [PubMed] [Google Scholar]
- Noguchi T., Fujiwara S. Development of glutamate:glyoxylate aminotransferase in the cotyledons of cucumber (Cucumis sativus) seedlings. Biochem J. 1982 Jan 1;201(1):209–214. doi: 10.1042/bj2010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Hayashi S. Peroxisomal localization and properties of tryptophan aminotransferase in plant leaves. J Biol Chem. 1980 Mar 25;255(6):2267–2269. [PubMed] [Google Scholar]
- Noguchi T., Hayashi S. Plant leaf alanine: 2-oxoglutarate aminotransferase. Peroxisomal localization and identity with glutamate:glyoxylate aminotransferase. Biochem J. 1981 Apr 1;195(1):235–239. doi: 10.1042/bj1950235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Purification and characterization of serine:glyoxylate aminotransferase from kidney bean (Phaseolus vulgaris). Biochim Biophys Acta. 1973 Sep 15;321(1):156–164. doi: 10.1016/0005-2744(73)90069-7. [DOI] [PubMed] [Google Scholar]
- Titus D. E., Hondred D., Becker W. M. Purification and characterization of hydroxypyruvate reductase from cucumber cotyledons. Plant Physiol. 1983 Jun;72(2):402–408. doi: 10.1104/pp.72.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]