Abstract
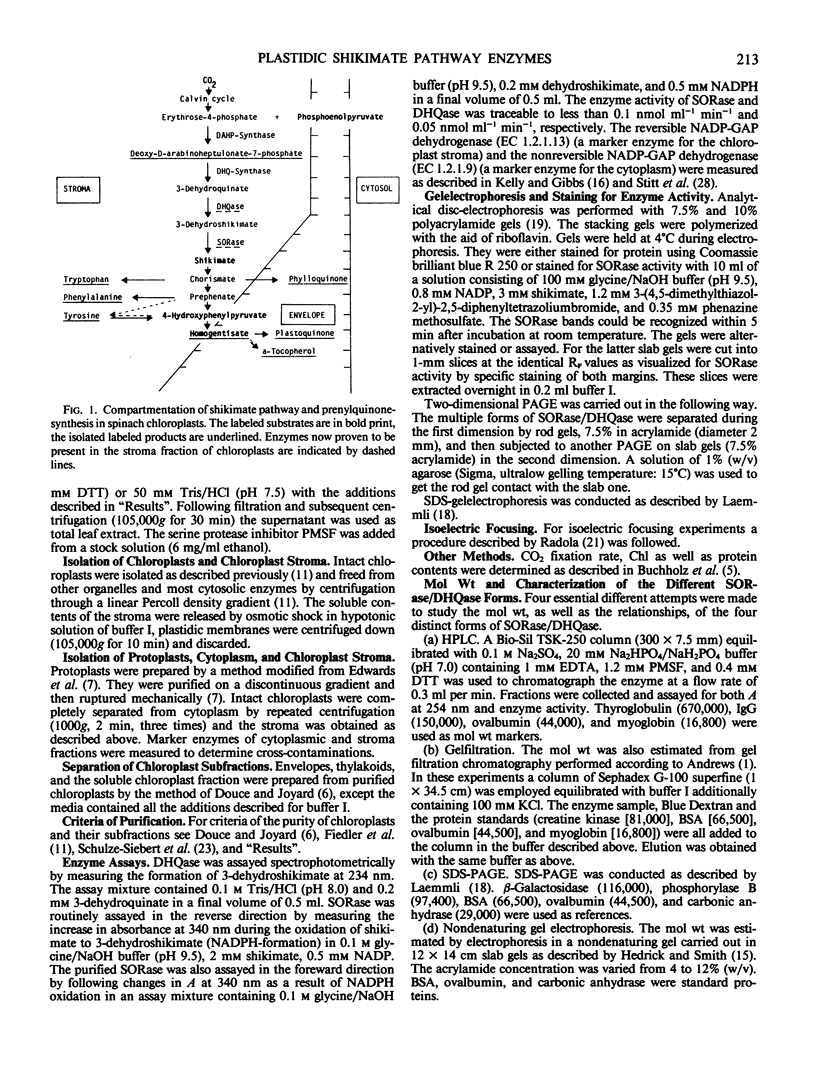
The stroma of chloroplasts is probably the sole site of the shikimate pathway enzymes shikimate oxidoreductase/dehydroquinate hydrolyase (SORase/DHQase) in spinach leaves. (a) The chromatographic behavior of the bifunctional protein SORase/DHQase on several separation materials with extracts from stroma compared with leaf extracts showed only one peak of enzymic activity originating from the stroma. (b) Polyacrylamide gel electrophoresis (PAGE) of these extracts followed by specific staining resulted in the same pattern without a band of extraplastidic enzyme. (c) In protoplast fractionation experiments it was shown that SORase/DHQase was present only in the soluble chloroplast protein fraction.
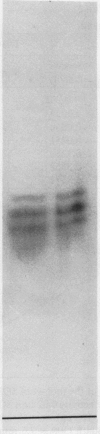
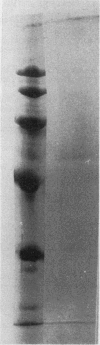
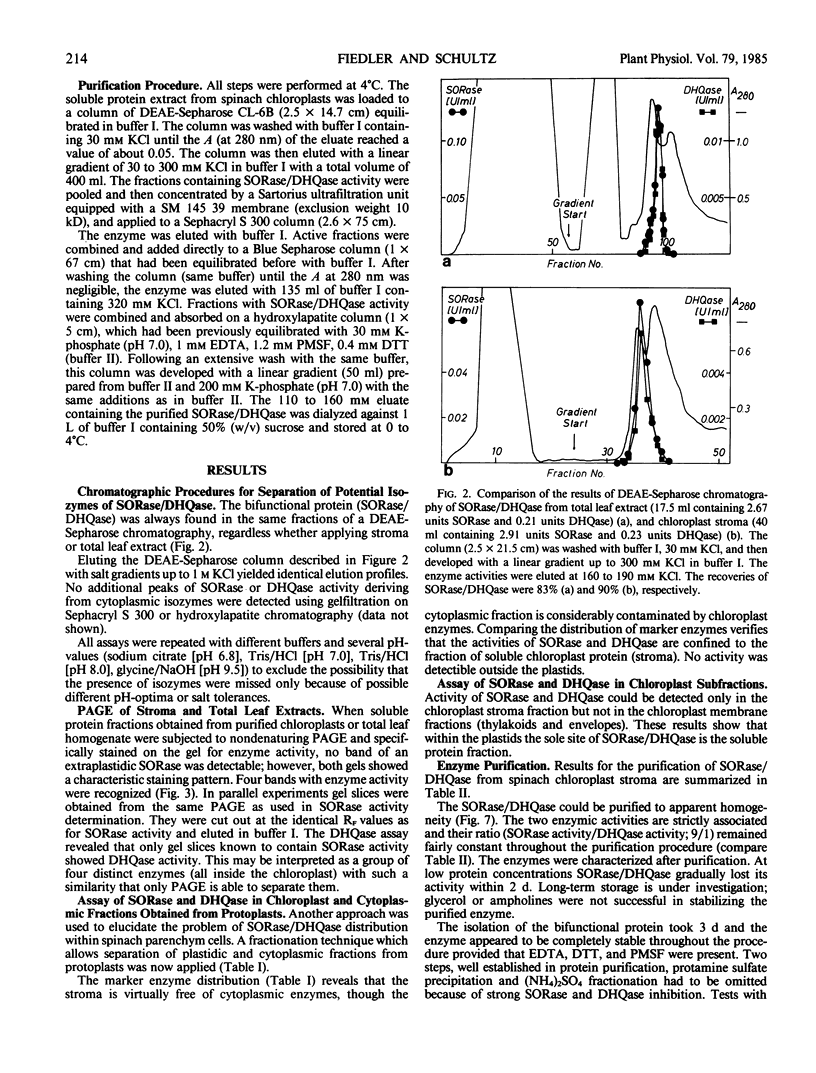
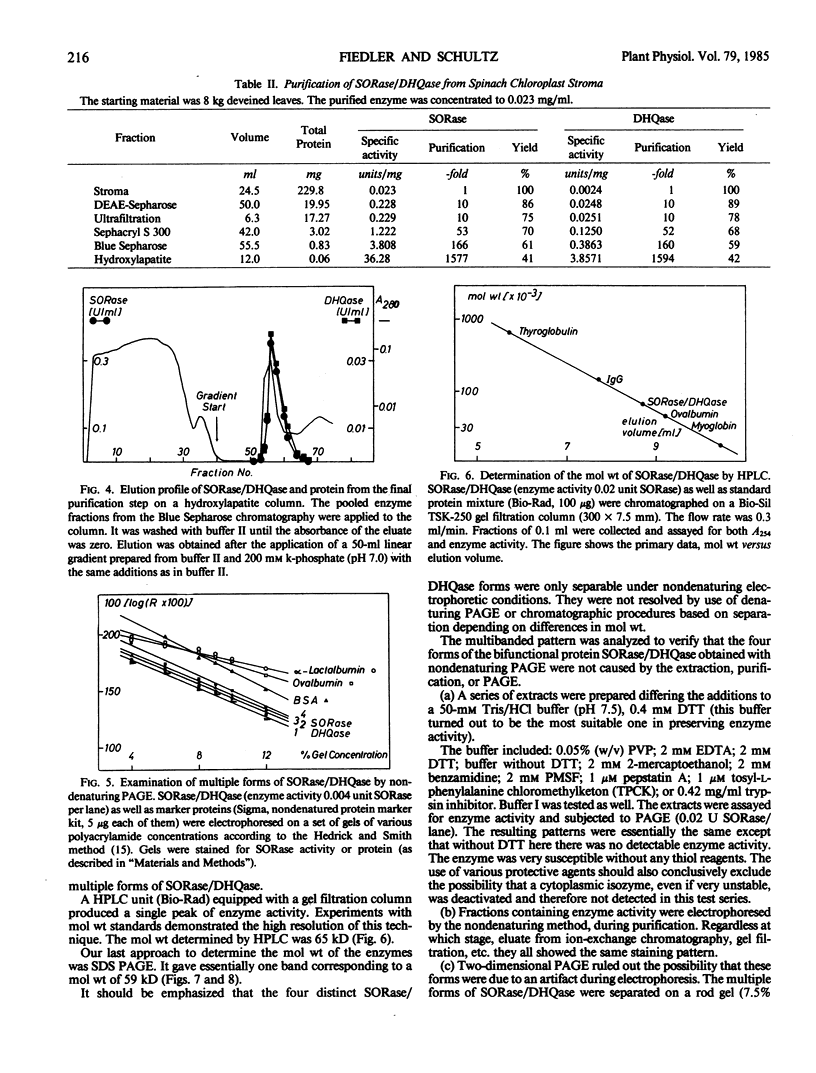
An improved purification procedure for SORase/DHQase from stroma of chloroplasts, yield 40%, 1600 times as pure, gave essentially one protein band on sodium dodecyl sulfate-PAGE. Our results demonstrate that both enzyme functions are carried out by a single polypeptide. Nondenaturing PAGE exhibited a pattern of four bands with SORase/DHQase showing that they differ in charge but not in their molecular weight. Molecular weight was determined to be 67 kilodaltons (gel filtration) and 59 kilodaltons (PAGE) for all four forms. It was proven they were not due to artifacts. The four forms show similar kinetic properties, their Km and pH optima differing only very slightly. Response to some metabolites is reported.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Polley L. D. Purification and characterization of 3-dehydroquinate hydrolase and shikmate oxidoreductase. Evidence for a bifunctional enzyme. Biochim Biophys Acta. 1978 Sep 11;526(1):259–266. doi: 10.1016/0005-2744(78)90310-8. [DOI] [PubMed] [Google Scholar]
- Schultz G., Ellerbrock B. H., Soll J. Site of prenylation reaction in synthesis of phylloquinone (vitamin K1) by spinach chloroplasts. Eur J Biochem. 1981 Jul;117(2):329–332. doi: 10.1111/j.1432-1033.1981.tb06341.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Siebert D., Heineke D., Scharf H., Schultz G. Pyruvate-Derived Amino Acids in Spinach Chloroplasts : Synthesis and Regulation during Photosynthetic Carbon Metabolism. Plant Physiol. 1984 Oct;76(2):465–471. doi: 10.1104/pp.76.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Kemmerling M., Schultz G. Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys. 1980 Oct 15;204(2):544–550. doi: 10.1016/0003-9861(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine biosynthesis in green leaves. Plant Physiol. 1983 Apr;71(4):780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]