Abstract
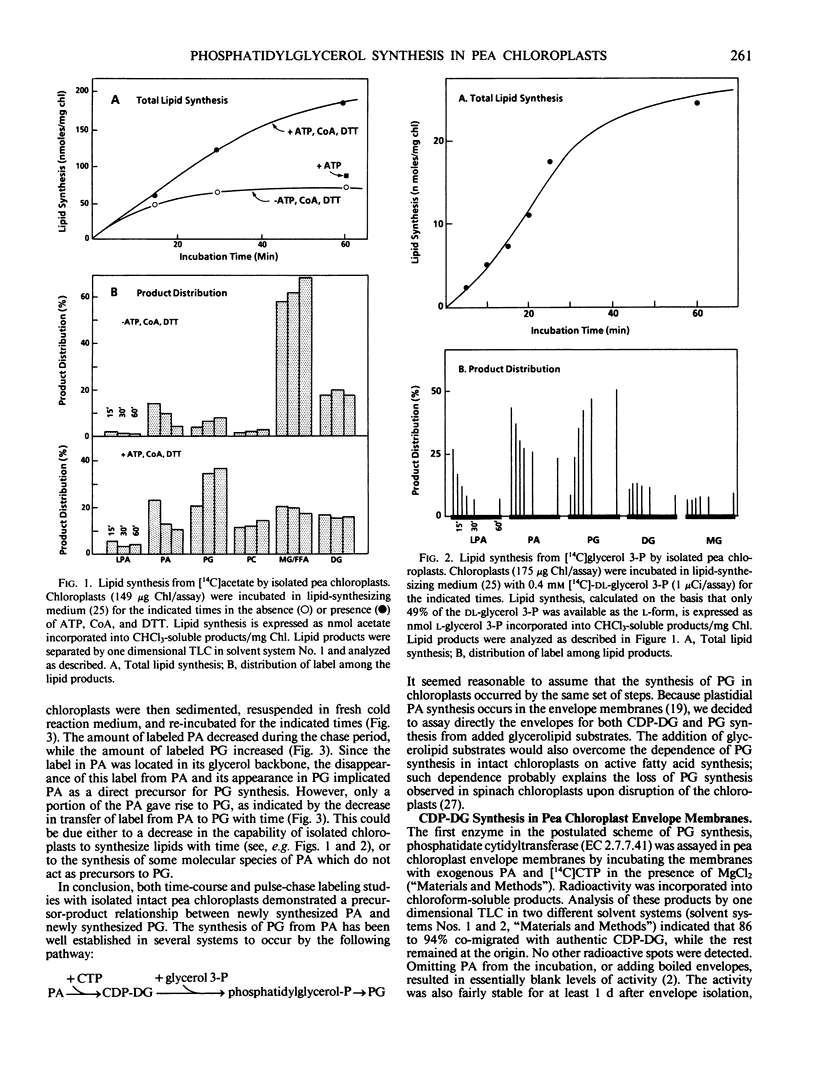
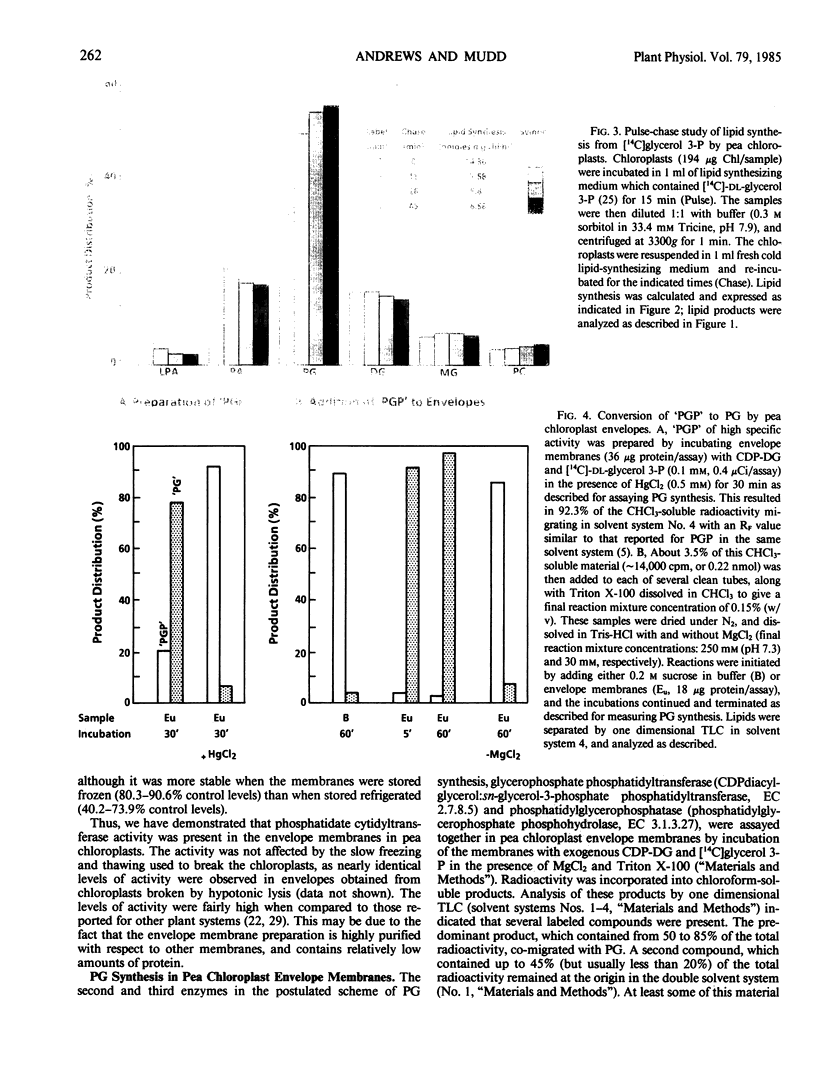
Isolated intact pea chloroplasts synthesized phosphatidylglycerol from either [14C]acetate or [14C]glycerol 3-phosphate. Both time-course and pulse-chase labeling studies demonstrated a precursor-product relationship between newly synthesized phosphatidic acid and newly synthesized phosphatidylglycerol.
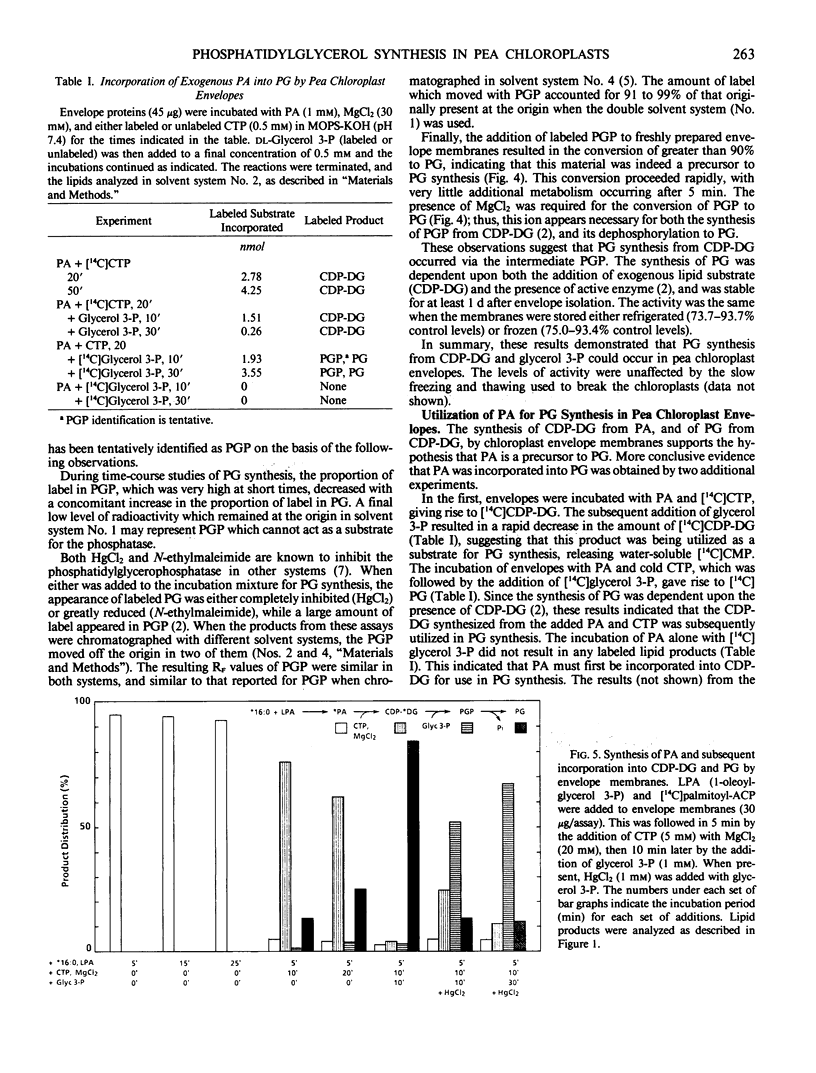
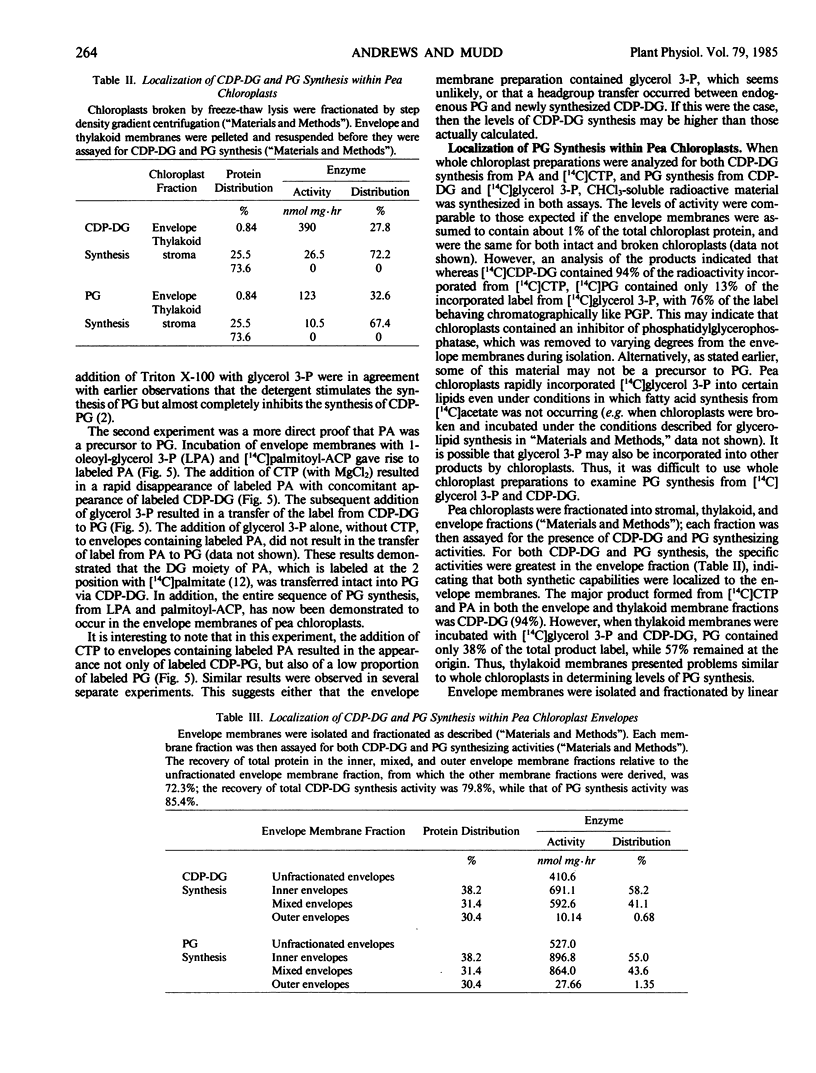
The synthesis both of CDP-diacylglycerol from exogenous phosphatidic acid and CTP, and of phosphatidylglycerol from exogenous CDP-diacylglycerol and glycerol 3-phosphate, could be assayed in fractions obtained from disrupted chloroplasts. Moreover, the enzymes catalyzing these reactions were localized in the inner envelope membrane. Exogenous phosphatidic acid was incorporated into phosphatidylglycerol, but only following its incorporation into CDP-diacylglycerol. Finally, radio-active phosphatidic acid synthesized in the envelope membranes from [14C]palmitoyl-ACP and 1-oleoyl-glycerol 3-phosphate was sequentially incorporated into labeled CDP-diacylglycerol and phosphatidylglycerol upon the addition of appropriate substrates and cofactors. Thus, we have demonstrated that (a) the synthesis of phosphatidylglycerol in chloroplasts occurs by the pathway: phosphatidic acid → CDP-diacylglycerol →→ phosphatidylglycerol, and (b) phosphatidylglycerol synthesis is located in the inner envelope membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J., Keegstra K. Acyl-CoA Synthetase Is Located in the Outer Membrane and Acyl-CoA Thioesterase in the Inner Membrane of Pea Chloroplast Envelopes. Plant Physiol. 1983 Jul;72(3):735–740. doi: 10.1104/pp.72.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Johnston J. M. CMP-dependent incorporation of [14C]Glycerol 3-phosphate into phosphatidylglycerol and phosphatidylglycerol phosphate by rabbit lung microsomes. Biochim Biophys Acta. 1982 Mar 12;710(3):377–390. doi: 10.1016/0005-2760(82)90121-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Phosphatidyl glycerophosphate phosphatase. J Lipid Res. 1967 Sep;8(5):456–462. [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983 Feb;71(2):366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Leonard J. M., Raetz C. R. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980 Feb 25;255(4):1623–1629. [PubMed] [Google Scholar]
- Haverkate F., van Deenen L. L. Isolation and chemical characterization of phosphatidyl glycerol from spinach leaves. Biochim Biophys Acta. 1965 Jul 7;106(1):78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kleppinger-Sparace K. F., Moore T. S. Biosynthesis of Cytidine 5'-Diphosphate-diacylglycerol in Endoplasmic Reticulum and Mitochondria of Castor Bean Endosperm. Plant Physiol. 1985 Jan;77(1):12–15. doi: 10.1104/pp.77.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of phosphatidylglycerol by cell-free preparations from spinach leaves. Biochim Biophys Acta. 1972 Apr 18;260(4):558–570. doi: 10.1016/0005-2760(72)90005-7. [DOI] [PubMed] [Google Scholar]
- McCaman R. E., Finnerty W. R. Biosynthesis of cytidine diphosphate-diglyceride by a particulate fracgion from Micrococcus cerificans. J Biol Chem. 1968 Oct 10;243(19):5074–5080. [PubMed] [Google Scholar]
- Mudd J. B., Dezacks R. Synthesis of phosphatidylglycerol by chloroplasts from leaves of Spinacia oleracea L. (spinach). Arch Biochem Biophys. 1981 Jul;209(2):584–591. doi: 10.1016/0003-9861(81)90316-7. [DOI] [PubMed] [Google Scholar]
- Sparace S. A., Mudd J. B. Phosphatidylglycerol synthesis in spinach chloroplasts: characterization of the newly synthesized molecule. Plant Physiol. 1982 Nov;70(5):1260–1264. doi: 10.1104/pp.70.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida S., Mudd J. B. The structure and biosynthesis of phosphatidyl inositol in cauliflower inflorescence. Plant Physiol. 1970 Jun;45(6):712–718. doi: 10.1104/pp.45.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]