Abstract
Microdroplets (3-5 nanoliters) of polyethylene glycol 8000 solution were allowed to equilibrate with plant water potential by placing the microdroplet on an abraded surface and covering it with mineral oil to prevent evaporation. Osmolality was followed by cryoscopic measurements, accurate to about ±0.1 bar, on subnanoliter samples.
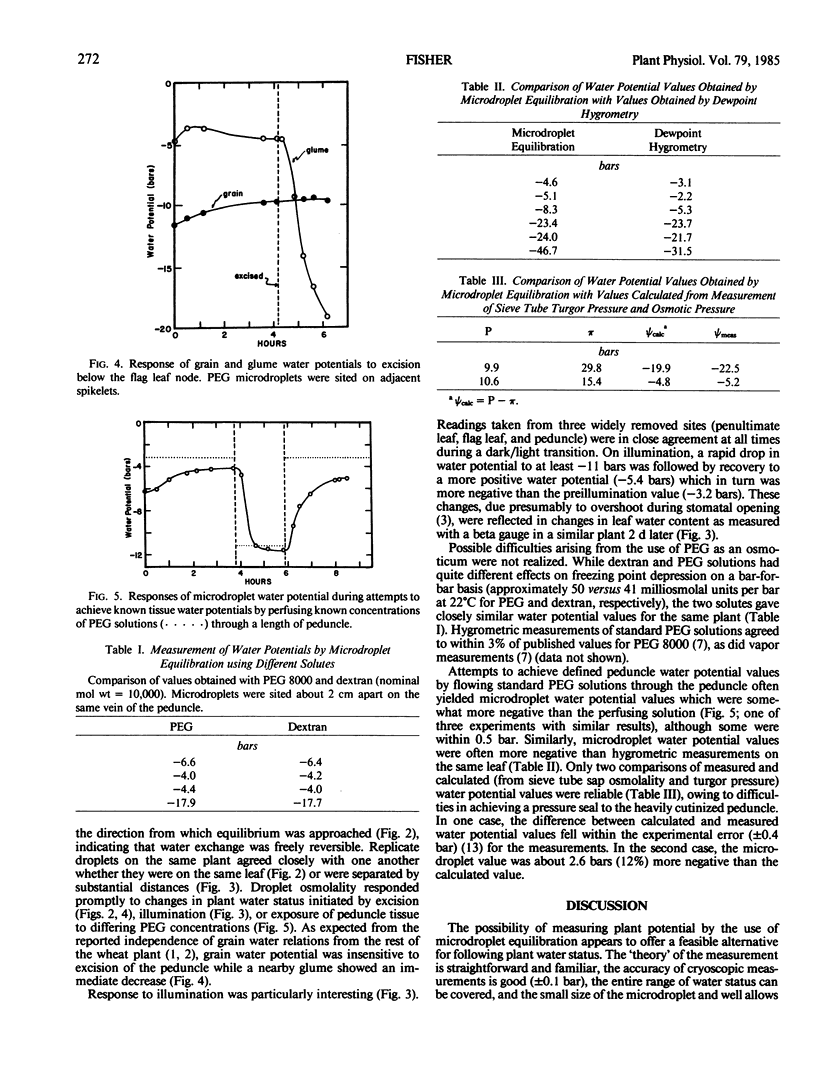
Under constant environmental conditions, apparent equilibrium between microdroplet and plant water potentials was attained in about 1 to 2 hours. Microdroplet osmolality responded promptly to treatments (illumination, excision, osmotica) which changed plant water status. The values obtained for plant water potentials appeared to be physiologically reasonable. However, comparison with values obtained by other means (dewpoint hygrometry, treatment of tissue with polyethylene glycol solutions, calculation from turgor and osmotic pressures) suggest that they might be somewhat more negative than the actual tissue water potential.
Aside from the advantage of providing in situ measurements of plant water status, the method is not temperature sensitive and requires only about 10 square millimeters of surface area, which allows its use on even small structures with little interference by shading or with gas exchange.
Full text
PDF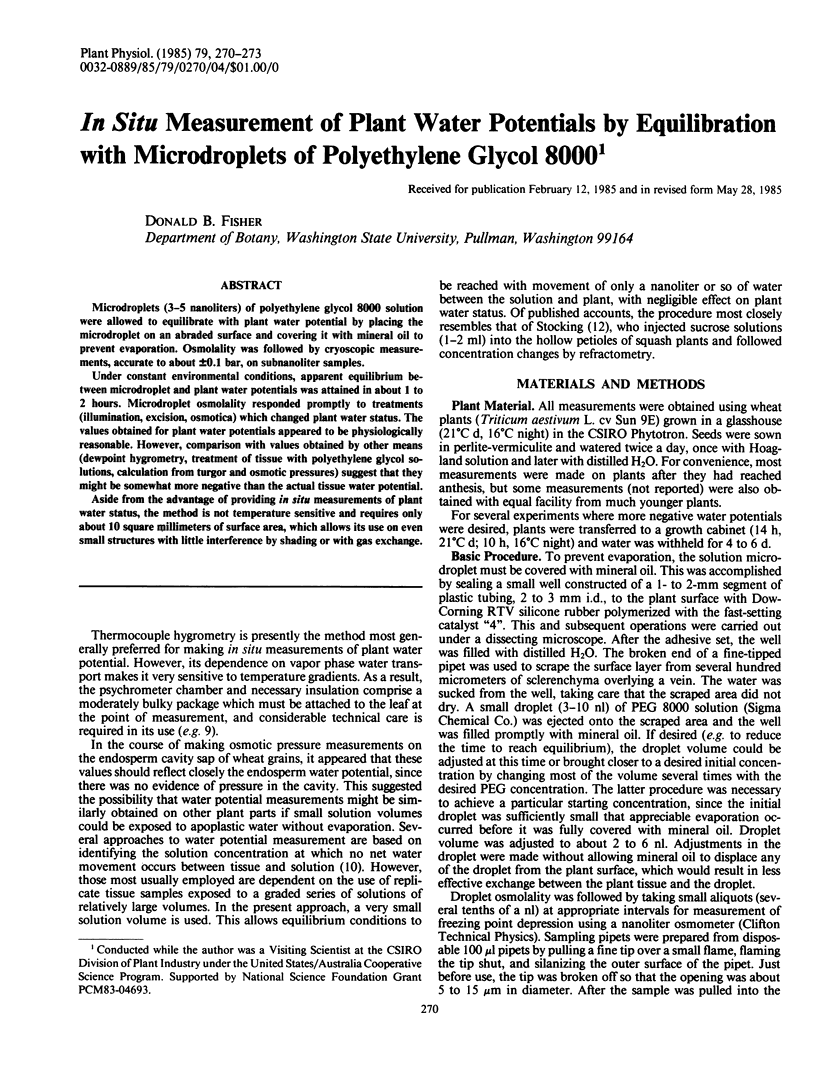


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Dreyer S. A., Seymour V., Cleland R. E. Low proton conductance of plant cuticles and its relevance to the Acid-growth theory. Plant Physiol. 1981 Sep;68(3):664–667. doi: 10.1104/pp.68.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E., Kaufmann M. R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973 May;51(5):914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C. E., Safir G. R., Hanson A. D. Water potential in excised leaf tissue: comparison of a commercial dew point hygrometer and a thermocouple psychrometer on soybean, wheat, and barley. Plant Physiol. 1978 Jan;61(1):131–133. doi: 10.1104/pp.61.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage M. J., Wiebe H. H., Cass A. In situ field measurement of leaf water potential using thermocouple psychrometers. Plant Physiol. 1983 Nov;73(3):609–613. doi: 10.1104/pp.73.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


