Abstract
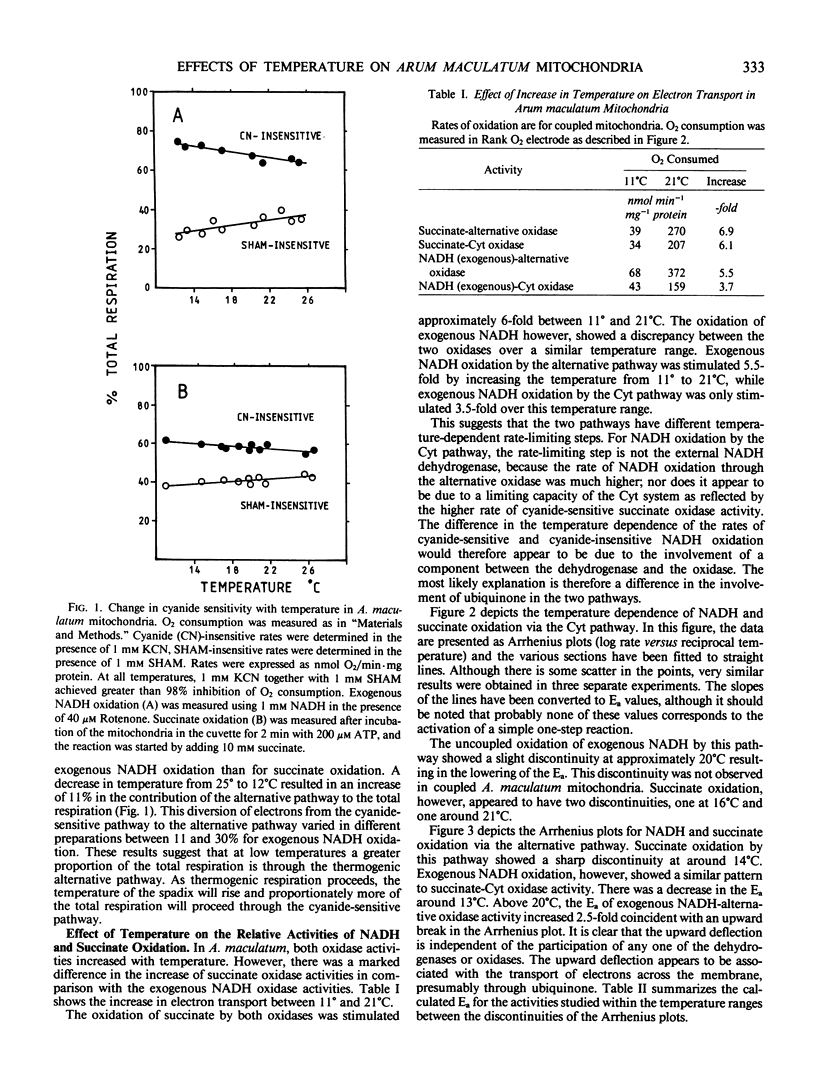
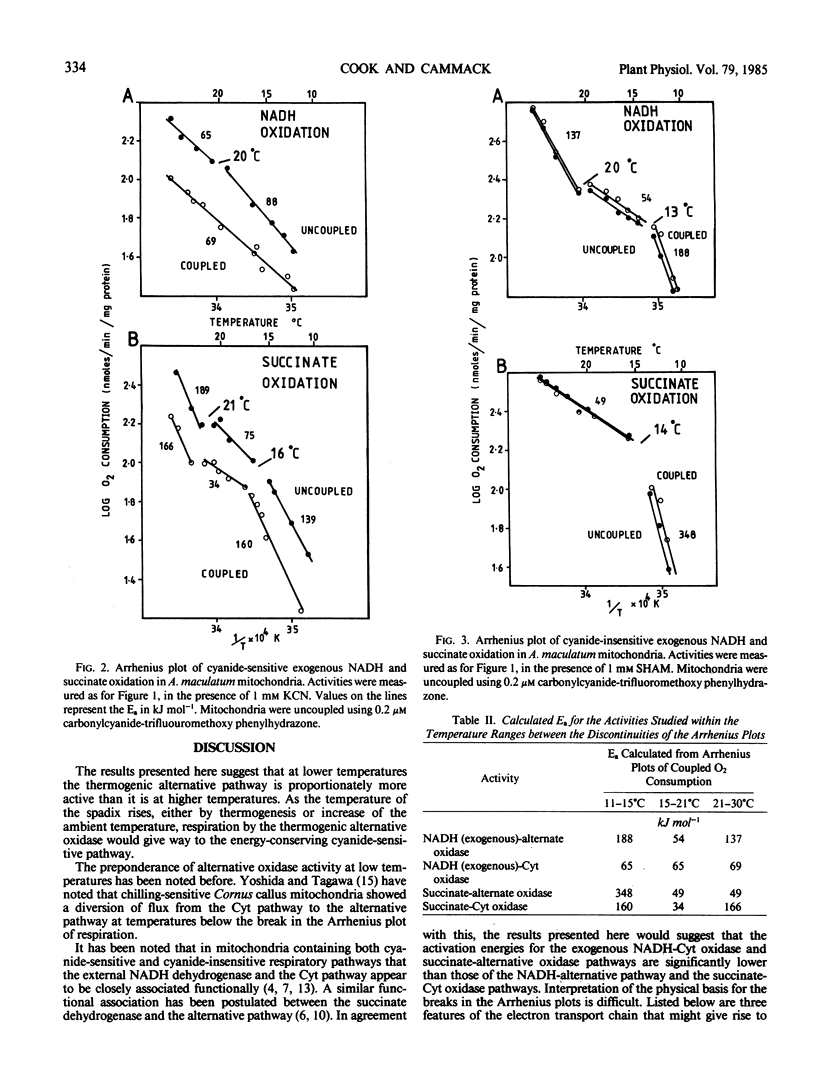
The effects of temperature upon the respiratory pathways of Arum maculatum mitochondria have been studied. The alternate oxidase sustained a greater proportion of the total respiration at low temperatures than at higher temperatures. Arrhenius plots of respiratory activities show two discontinuities, one at 14°C and one at 21°C. The lower temperature discontinuity was associated with electron transport from succinate dehydrogenase to the alternative oxidase, enzymes that face the inner side of the membrane while the higher temperature discontinuity was associated with electron transport from the external NADH dehydrogenase to cytochrome c oxidase, which face the outer side of the membrane. Both discontinuities resulted in a decrease in the activation energy for electron transport on one side of the membrane. Arrhenius plots of transmembrane electron transport showed discontinuities at both 14° and 21°C but the upper discontinuity resulted in an increase in the activation energy. Activation energies determined for the respiratory activities show that above 21°C the exogenous NADH-cytochrome pathway and the succinate-alternative oxidase pathway were lower than those for the NADH-alternative pathway or the succinate cytochrome pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cammack R., Palmer J. M. Iron-sulphur centres in mitochondria from Arum maculatum spadix with very high rates of cyanide-resistant respiration. Biochem J. 1977 Sep 15;166(3):347–355. doi: 10.1042/bj1660347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Rich P. R., Bonner W. D., Jr, Ingledew W. J. A complex EPR signal in mung bean mitochondria and its possible relation to the alternate pathway. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1099–1107. doi: 10.1016/s0006-291x(76)80245-8. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. K. The effect of nucleotides and inhibitors on respiration in isolated wheat mitochondria. Plant Physiol. 1975 Jan;55(1):51–58. doi: 10.1104/pp.55.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Non-linear Arrhenius plots and the analysis of reaction and motional rates in biological membranes. J Theor Biol. 1981 Jan 7;88(1):135–152. doi: 10.1016/0022-5193(81)90332-5. [DOI] [PubMed] [Google Scholar]
- Thilo L., Träuble H., Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977 Apr 5;16(7):1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]
- Tomlinson P. F., Moreland D. E. Cyanide-resistant Respiration of Sweet Potato Mitochondria. Plant Physiol. 1975 Feb;55(2):365–369. doi: 10.1104/pp.55.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


