Abstract
The effects of water deficits on plant morphology and biochemistry were analyzed in two photoperiodic strains of field-grown cotton (Gossypium hirsutum L.). Plants grown under dryland conditions exhibited a 40 to 85% decrease in leaf number, leaf area index, leaf size, plant height, and total weight per plant. Gross photosynthesis decreased from 0.81 to 0.47 milligram CO2 fixed per meter per second and the average midday water, osmotic, and turgor potentials decreased to −2.1, −2.4, and 0.3 megapascals, respectively.
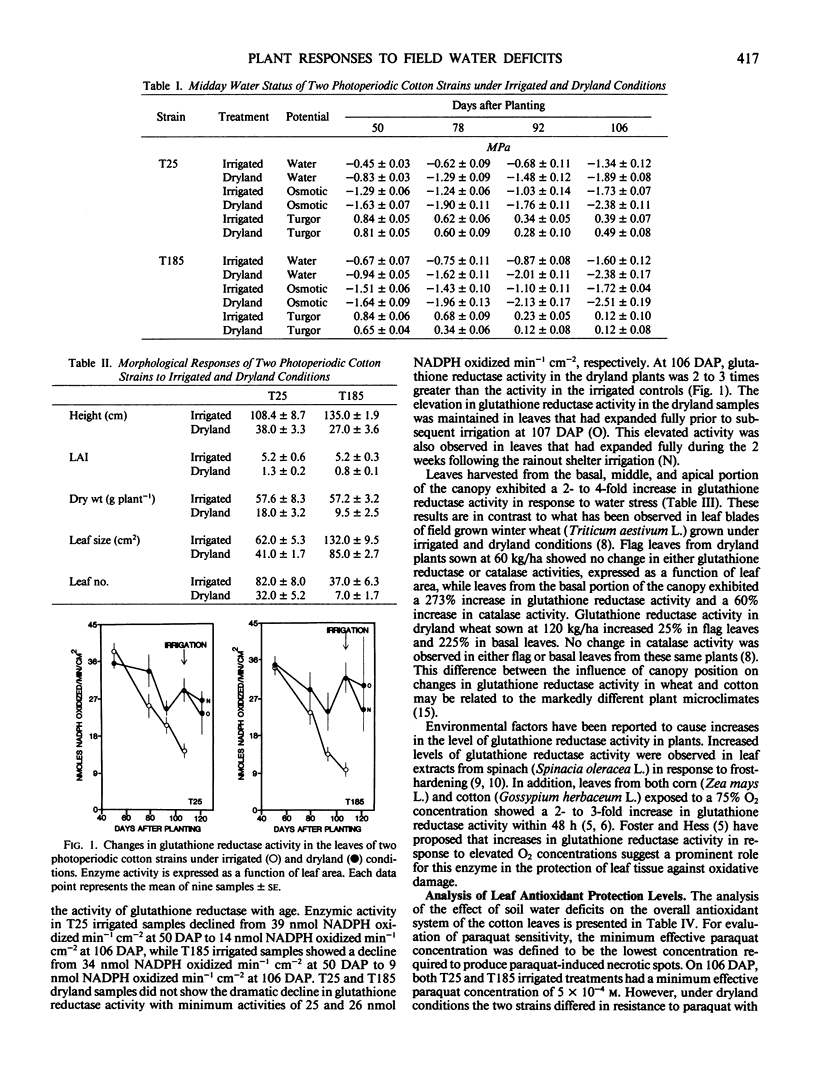
There was a progressive increase in glutathione reductase activity and in the cellular antioxidant system in the leaves of stressed plants compared to the irrigated controls. The stress-induced increases in enzyme activity occurred at all canopy positions analyzed.
Irrigation of the dryland plots following severe water stress resulted in a 50% increase in leaf area per gram fresh weight in newly expanded leaves of both strains over the leaves which had expanded under the dryland conditions. Paraquat resistance (a relative measure of the cellular antioxidant system) decreased in the strain T25 following irrigation. Glutathione reductase activities remained elevated in the T25 and T185 leaves which were expanded fully prior to irrigation and in the leaves which expanded following the irrigation treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderbank A. The bipyridylium herbicides. Adv Pest Control Res. 1968;8:127–235. [PubMed] [Google Scholar]
- Cowan I. R., Farquhar G. D. Stomatal function in relation to leaf metabolism and environment. Symp Soc Exp Biol. 1977;31:471–505. [PubMed] [Google Scholar]
- Dodge A. D. The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavour. 1971 Sep;30(111):130–135. doi: 10.1016/0160-9327(71)90039-1. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. G., Hess J. L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980 Sep;66(3):482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Burke J. J. Effect of water stress on the chloroplast antioxidant system: I. Alterations in glutathione reductase activity. Plant Physiol. 1984 Nov;76(3):615–621. doi: 10.1104/pp.76.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Saetre R., Rabenstein D. L. Determination of cysteine and glutathione in fruit by high-performance liquid chromatography. J Agric Food Chem. 1978 Jul-Aug;26(4):982–983. doi: 10.1021/jf60218a028. [DOI] [PubMed] [Google Scholar]


