Abstract
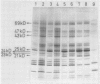
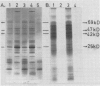
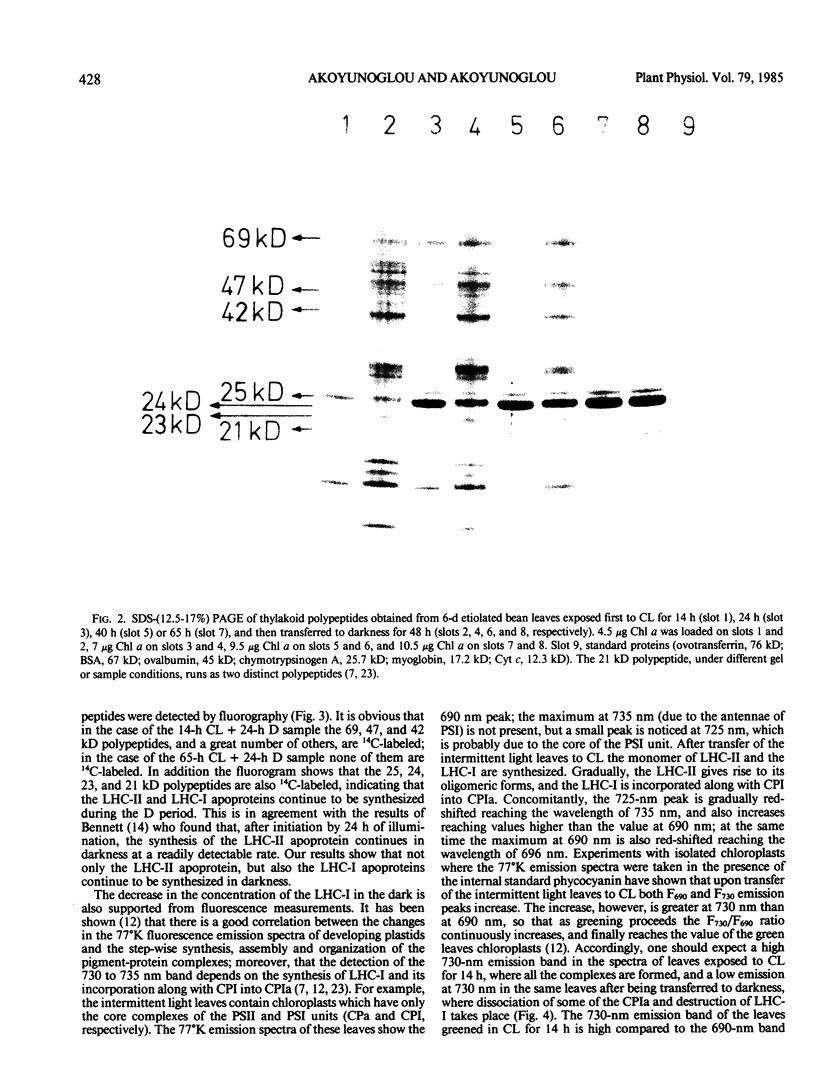
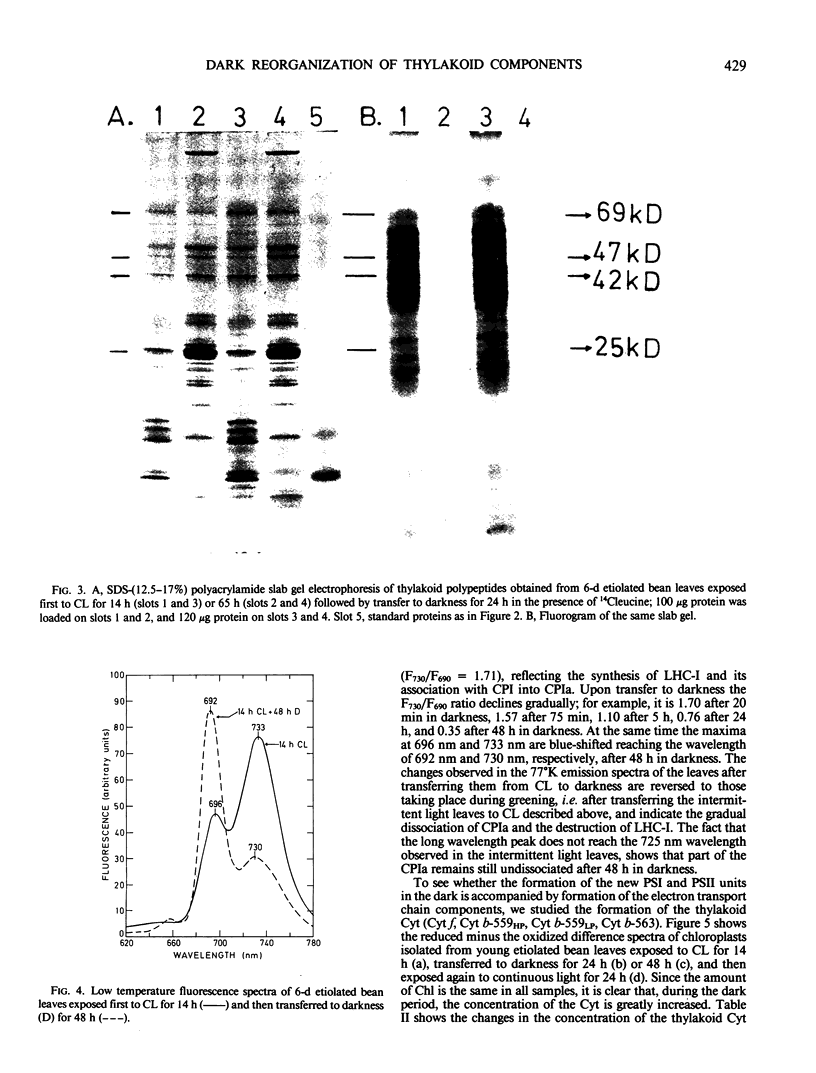
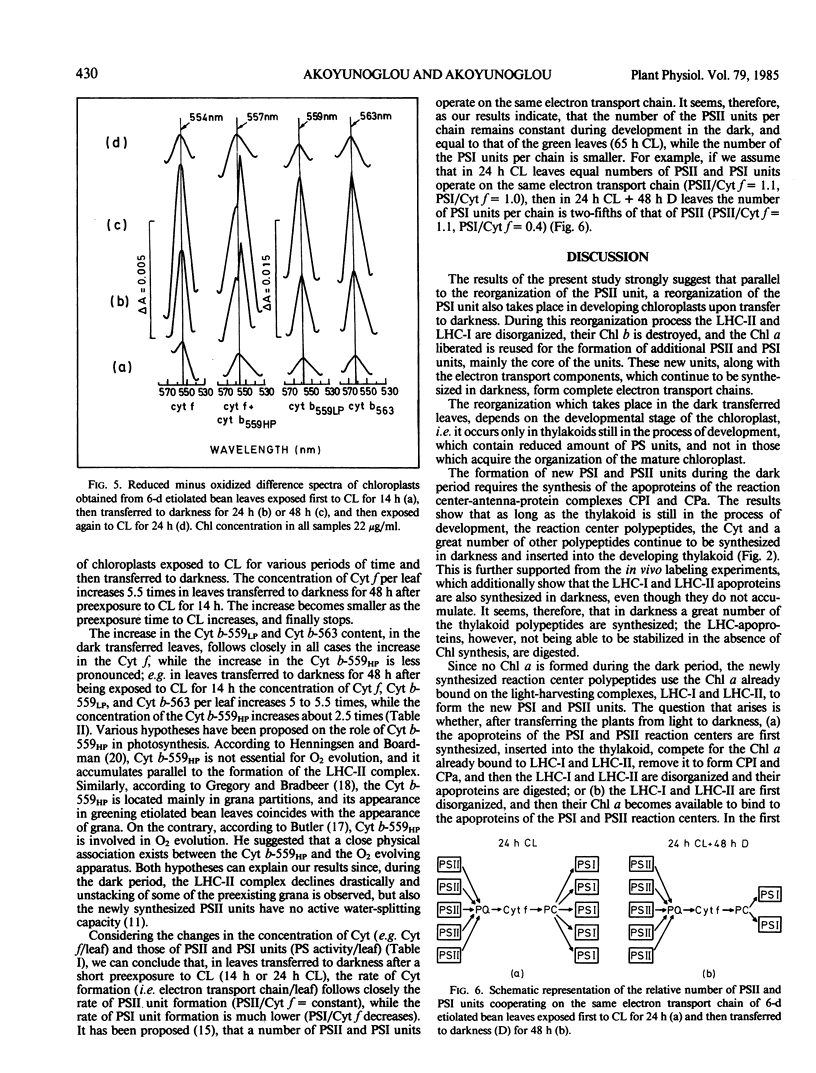
It was shown earlier that in etiolated bean (Phaseolus vulgaris, var. red kidney) leaves exposed to continuous light for a short time and then transferred to darkness a reorganization of their photosystem II (PSII) unit components occurs. This reorganization involves disorganization of the light-harvesting complex of PSII (LHC-II), destruction of its chlorophyll b and the 25 kilodalton polypeptide, and reuse of its chlorophyll a for the formation of additional, small in size, PSII units (Argyroudi-Akoyunoglou, Akoyunoglou, Kalosakas, Akoyunoglou 1982 Plant Physiol 70: 1242-1248). The present study further shows that parallel to the PSII unit reorganization a reorganization of the PSI unit components also occurs: upon transfer to darkness the 24, 23, and 21 kilodalton polypeptides, components of the light-harvesting complex of PSI (LHC-I), are decreased, the 69 kilodalton polypeptide, component of the chlorophyll a-rich P700-protein complex (CPI), is increased and new smallsized PSI units are formed. Concomitantly, the cytochrome f/chlorophyll and the cytochrome b/chlorophyll ratios are gradually increased. This suggests that the concentration of the electron transport components is also modulated in darkness to allow for adequate electron flow to occur between the newly synthesized PSII and PSI units.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akoyunoglou G. Development of the photosystem II unit in plastids of bean leaves greened in periodic light. Arch Biochem Biophys. 1977 Oct;183(2):571–580. doi: 10.1016/0003-9861(77)90392-7. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou A., Kalosakas K., Akoyunoglou G. Reorganization of the Photosystem II Unit in Developing Thylakoids of Higher Plants after Transfer to Darkness : Changes in Chlorophyll b, Light-Harvesting Chlorophyll Protein Content, and Grana Stacking. Plant Physiol. 1982 Nov;70(5):1242–1248. doi: 10.1104/pp.70.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Correlation between cation-induced formation of heavy subchloroplast fractions and cation-induced increase in chlorophyll a fluorescence yield in tricine-washed chloroplasts. Arch Biochem Biophys. 1977 Mar;179(2):370–377. doi: 10.1016/0003-9861(77)90124-2. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Photoinduced changes in the chlorophyll a to chlorophyll B ratio in young bean plants. Plant Physiol. 1970 Aug;46(2):247–249. doi: 10.1104/pp.46.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H. Effect of cations on the reconstitution of heavy subchloroplast fractions (grana) in disorganized low-salt agranal chloroplasts. Arch Biochem Biophys. 1976 Sep;176(1):267–274. doi: 10.1016/0003-9861(76)90165-x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Reeves S. G., Baltscheffsky H. Photosynthetic control in isolated spinach chloroplasts with endogenous and artificial electron acceptors. Biochem Biophys Res Commun. 1971 Apr 16;43(2):359–366. doi: 10.1016/0006-291x(71)90761-3. [DOI] [PubMed] [Google Scholar]
- Henningsen K. W., Boardman N. K. Development of Photochemical Activity and the Appearance of the High Potential Form of Cytochrome b-559 in Greening Barley Seedlings. Plant Physiol. 1973 Jun;51(6):1117–1126. doi: 10.1104/pp.51.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Millington R. H., D'Angelo L. P. Structural similarities between the major polypeptides of thylakoid membranes from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1980 Jun;202(1):221–234. doi: 10.1016/0003-9861(80)90424-5. [DOI] [PubMed] [Google Scholar]
- Kuang T. Y., Argyroudi-Akoyunoglou J. H., Nakatani H. Y., Watson J., Arntzen C. J. The origin of the long-wavelength fluorescence emission band (77 degrees K) from photosystem I. Arch Biochem Biophys. 1984 Dec;235(2):618–627. doi: 10.1016/0003-9861(84)90236-4. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Thornber J. P., Skrdla M. P. Effect of detergents on the reliability of a chemical assay for P-700. Biochim Biophys Acta. 1980 Jul 8;591(2):391–399. doi: 10.1016/0005-2728(80)90170-x. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. The redox potentials of the b-type cytochromes of higher plant chloroplasts. Biochim Biophys Acta. 1980 Jun 10;591(1):153–161. doi: 10.1016/0005-2728(80)90229-7. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: a photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969 Nov;44(11):1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]