Abstract
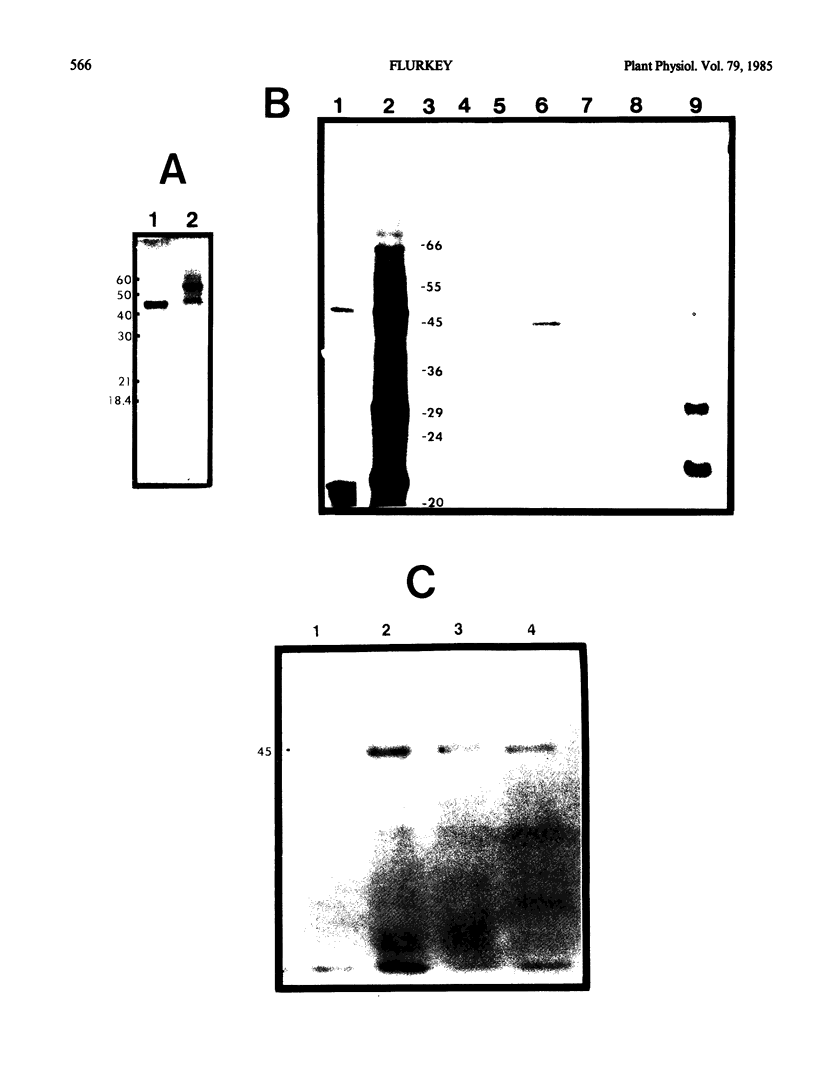
Poly A+ mRNA was isolated from Vicia faba leaves and translated in vitro using a rabbit reticulocyte translation system. From analysis of the total translation products, the major proteins synthesized in vitro were 32 kilodaltons and 20 kilodaltons. When antibodies to Vicia faba polyphenoloxidase were added, a specific immunoprecipitable protein was observed. This protein's molecular weight was shown to be similar to that of the isolated enzyme (45 kilodaltons). The isolated enzyme successfully competed with the in vitro synthesized product for antipolyphenoloxidase. In addition, the in vitro synthesized product was not immunoprecipitated with antitomato peroxidase and comigrated with isolated and/or iodinated enzyme in sodium dodecylsulfate-polyacrylamide gel electrophoresis. Using in vitro translation and specific immunoprecipitation, a primary translation product corresponding to Vicia faba polyphenoloxidase was identified as a 45 kilodaltons protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey W. H., Kolattukudy P. E. In vitro translation of cutinase mRNA: evidence for a precursor form of an extracellular fungal enzyme. Arch Biochem Biophys. 1981 Nov;212(1):154–161. doi: 10.1016/0003-9861(81)90354-4. [DOI] [PubMed] [Google Scholar]
- Flurkey W., Kim Y. S., Kolattukudy P. E. Precursor of a mitochondrial enzyme accumulates in the cytoplasm of preen glands for a specialized function. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1346–1352. doi: 10.1016/0006-291x(82)91261-x. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Hutcheson S. W., Buchanan B. B. Polyphenol Oxidation by Vicia faba Chloroplast Membranes: STUDIES ON THE LATENT MEMBRANE-BOUND POLYPHENOL OXIDASE AND ON THE MECHANISM OF PHOTOCHEMICAL POLYPHENOL OXIDATION. Plant Physiol. 1980 Dec;66(6):1150–1154. doi: 10.1104/pp.66.6.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury H. J., Possingham J. V. Factors affecting the extraction of intact ribonucleic Acid from plant tissues containing interfering phenolic compounds. Plant Physiol. 1977 Oct;60(4):543–547. doi: 10.1104/pp.60.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]