Abstract
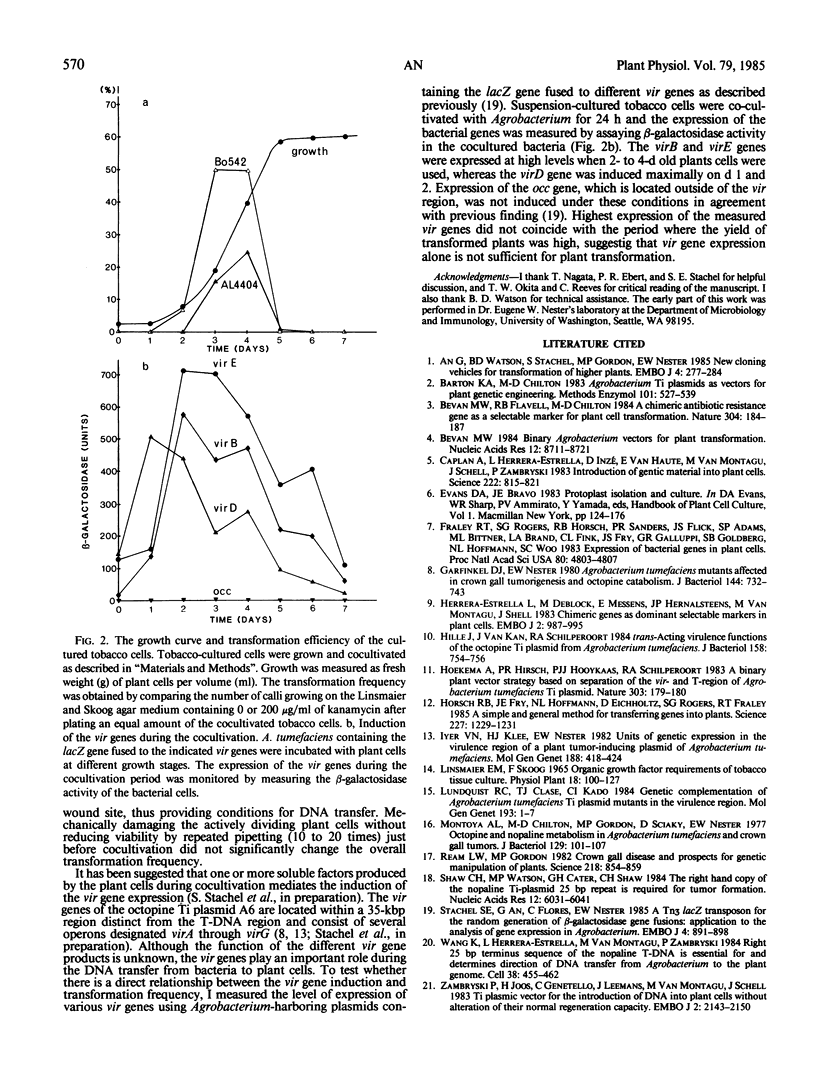
Tobacco calli were transformed at levels up to 50% by cocultivation of tobacco cultured cells with Agrobacterium tumefaciens harboring the binary transfer-DNA vector, pGA472, containing a kanamycin resistance marker. Transformation frequency was dependent on the physiological state of the tobacco cells, the nature of Agrobacterium strain and, less so, on the expression of the vir genes of the tumor-inducing plasmid. Maximum transformation frequency was obtained with exponentially growing plant cells, suggesting that rapid growth of plant cells is an essental factor for efficient transformation of higher plants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- An G., Watson B. D., Stachel S., Gordon M. P., Nester E. W. New cloning vehicles for transformation of higher plants. EMBO J. 1985 Feb;4(2):277–284. doi: 10.1002/j.1460-2075.1985.tb03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K. A., Chilton M. D. Agrobacterium Ti plasmids as vectors for plant genetic engineering. Methods Enzymol. 1983;101:527–539. doi: 10.1016/0076-6879(83)01036-8. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A., Herrera-Estrella L., Inzé D., Van Haute E., Van Montagu M., Schell J., Zambryski P. Introduction of genetic material into plant cells. Science. 1983 Nov 18;222(4625):815–821. doi: 10.1126/science.222.4625.815. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Block M. D., Messens E., Hernalsteens J. P., Montagu M. V., Schell J. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 1983;2(6):987–995. doi: 10.1002/j.1460-2075.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille J., van Kan J., Schilperoort R. trans-Acting virulence functions of the octopine Ti plasmid from Agrobacterium tumefaciens. J Bacteriol. 1984 May;158(2):754–756. doi: 10.1128/jb.158.2.754-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N., Klee H. J., Nester E. W. Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):418–424. doi: 10.1007/BF00330043. [DOI] [PubMed] [Google Scholar]
- Lundquist R. C., Close T. J., Kado C. I. Genetic complementation of Agrobacterium tumefaciens Ti plasmid mutants in the virulence region. Mol Gen Genet. 1984;193(1):1–7. doi: 10.1007/BF00327406. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream L. W., Gordon M. P. Crown gall disease and prospects for genetic manipulation of plants. Science. 1982 Nov 26;218(4575):854–859. doi: 10.1126/science.218.4575.854. [DOI] [PubMed] [Google Scholar]
- Shaw C. H., Watson M. D., Carter G. H., Shaw C. H. The right hand copy of the nopaline Ti-plasmid 25 bp repeat is required for tumour formation. Nucleic Acids Res. 1984 Aug 10;12(15):6031–6041. doi: 10.1093/nar/12.15.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Herrera-Estrella L., Van Montagu M., Zambryski P. Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from agrobacterium to the plant genome. Cell. 1984 Sep;38(2):455–462. doi: 10.1016/0092-8674(84)90500-2. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



