Abstract
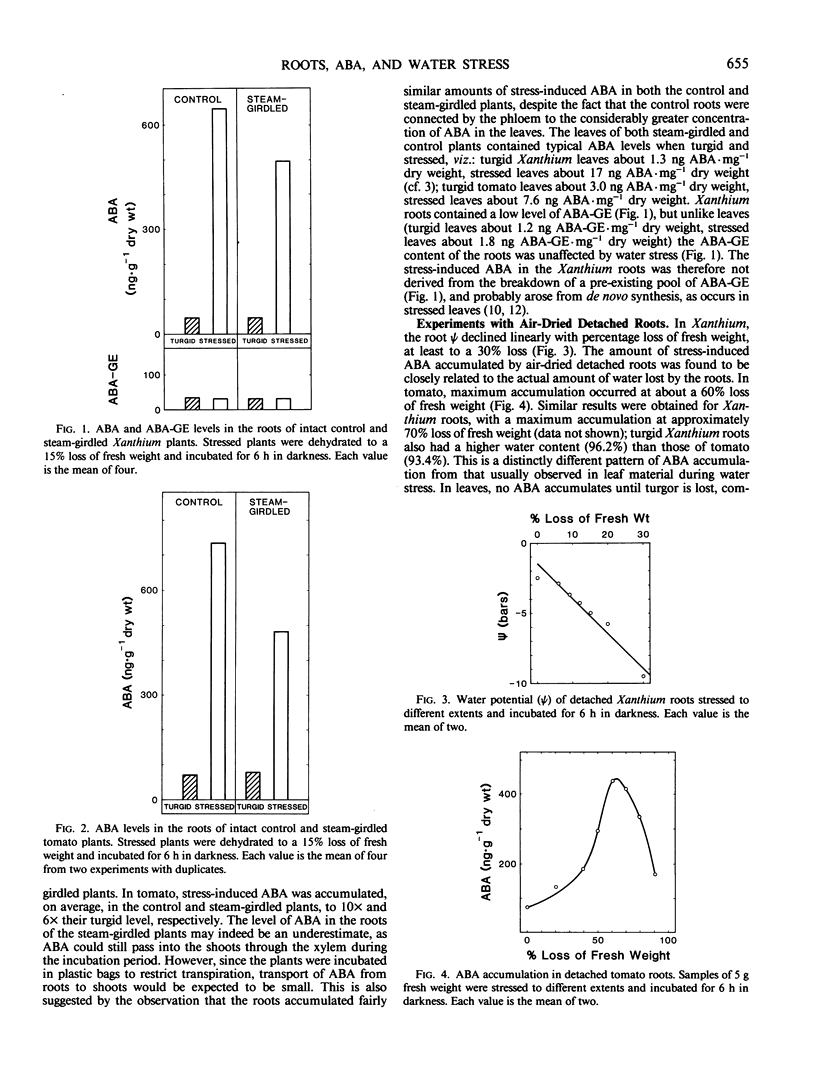
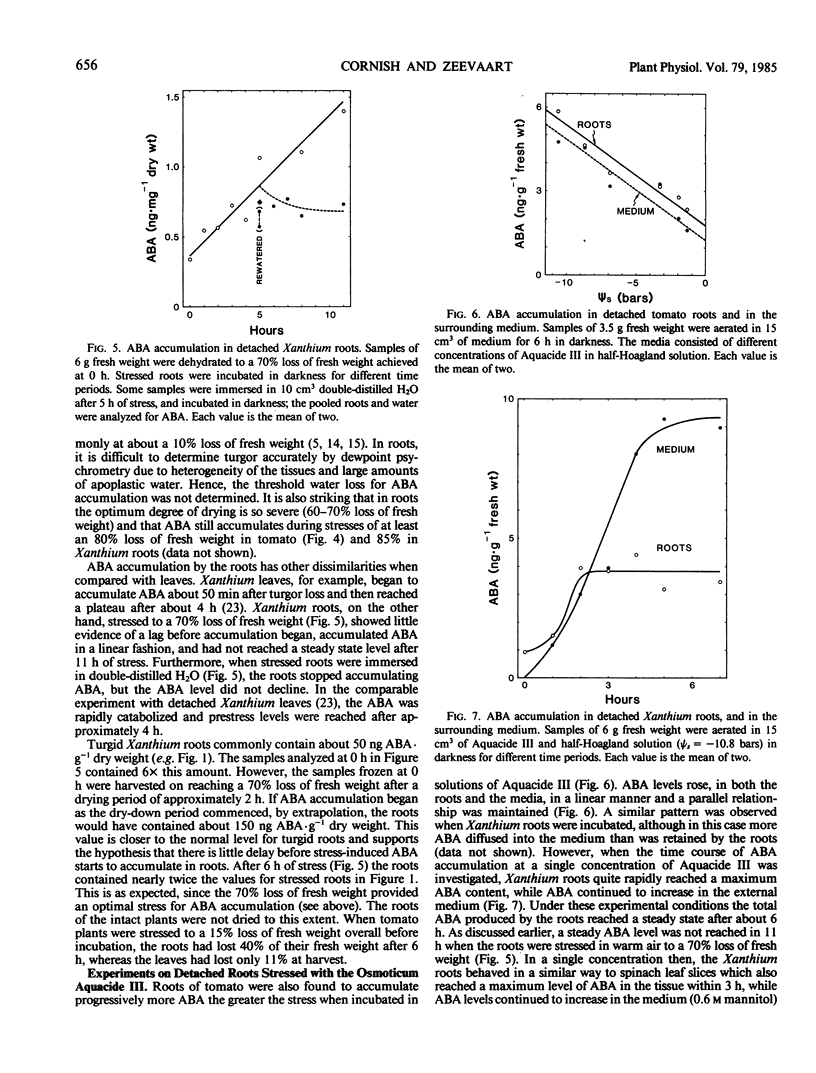
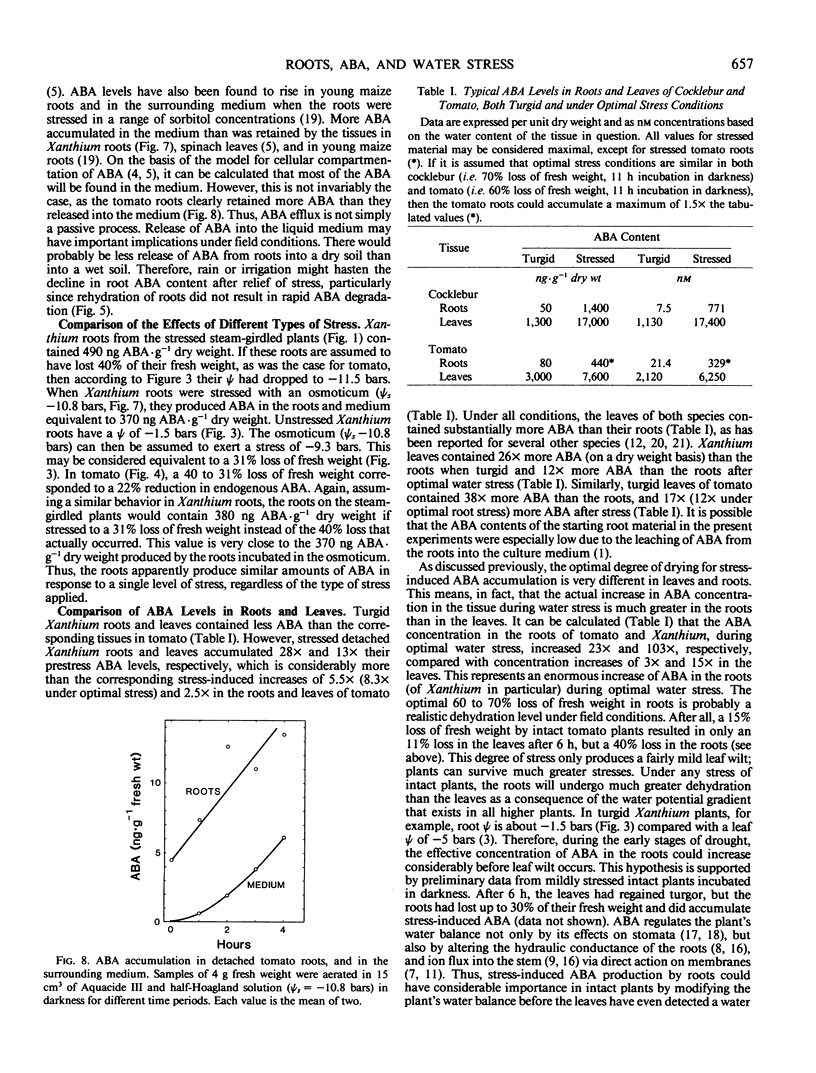
Plants of Xanthium strumarium L. and Lycopersicon esculentum Mill. cv `Rheinlands Ruhm' were grown in solution culture, and control and steam-girdled intact plants were stressed. Detached roots of both species were stressed to different extents in two ways: (a) either in warm air or, (b) in the osmoticum Aquacide III. The roots of both species produced and accumulated progressively more abscisic acid (ABA), the greater the stress inflicted by either method. ABA-glucose ester levels in Xanthium roots were not affected by water stress and were too low to be the source of the stress-induced ABA. The fact that ABA accumulated in detached roots and in roots of girdled plants proves that ABA was synthesized in the roots and not merely transported from the shoots.
Maximum ABA accumulation in detached roots occurred after 60 to 70% loss of fresh weight. In Xanthium roots, ABA levels continued to increase for at least 11 hours, and no catabolism was apparent when stressed roots were immersed in water, although the roots did stop accumulating ABA. When osmotically stressed, Xanthium roots reached a maximum ABA level after 2 hours, but ABA continued to rise in the medium.
Under optimal stress conditions, endogenous ABA levels increased 100 times over their prestress values in detached roots of Xanthium, and 15 times in Lycopersicon under nonoptimal stress, when endogenous ABA was expressed as concentrations based on tissue water content. These are much greater relative increases than observed in the leaves (15 times in Xanthium, 3 times in Lycopersicon), although the roots contain substantially less ABA than the leaves in all circumstances. The results suggest that the endogenous level of ABA in roots could rise appreciably prior to leaf wilt, and could modify the plant's water economy before the leaves become stressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsell M. F., Cohen D. Relationships between Leaf Water Status, Abscisic Acid Levels, and Stomatal Resistance in Maize and Sorghum. Plant Physiol. 1975 Aug;56(2):207–212. doi: 10.1104/pp.56.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Metabolism in Relation to Water Stress and Leaf Age in Xanthium strumarium. Plant Physiol. 1984 Dec;76(4):1029–1035. doi: 10.1104/pp.76.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Abscisic Acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol. 1985 Jan;77(1):25–28. doi: 10.1104/pp.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus E. L. Effects of Abscisic Acid on the Hydraulic Conductance of and the Total Ion Transport through Phaseolus Root Systems. Plant Physiol. 1981 Jul;68(1):169–174. doi: 10.1104/pp.68.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markhart A. H., Fiscus E. L., Naylor A. W., Kramer P. J. Effect of abscisic Acid on root hydraulic conductivity. Plant Physiol. 1979 Oct;64(4):611–614. doi: 10.1104/pp.64.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C. E., Safir G. R., Hanson A. D. Water potential in excised leaf tissue: comparison of a commercial dew point hygrometer and a thermocouple psychrometer on soybean, wheat, and barley. Plant Physiol. 1978 Jan;61(1):131–133. doi: 10.1104/pp.61.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. Water Relations of Cotton Plants under Nitrogen Deficiency: III. STOMATAL CONDUCTANCE, PHOTOSYNTHESIS, AND ABSCISIC ACID ACCUMULATION DURING DROUGHT. Plant Physiol. 1981 Jan;67(1):115–119. doi: 10.1104/pp.67.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A., Boyer G. L. Accumulation and transport of abscisic Acid and its metabolites in ricinus and xanthium. Plant Physiol. 1984 Apr;74(4):934–939. doi: 10.1104/pp.74.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A., Brede J. M., Cetas C. B. Translocation patterns in xanthium in relation to long day inhibition of flowering. Plant Physiol. 1977 Nov;60(5):747–753. doi: 10.1104/pp.60.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


