Abstract
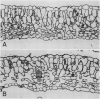
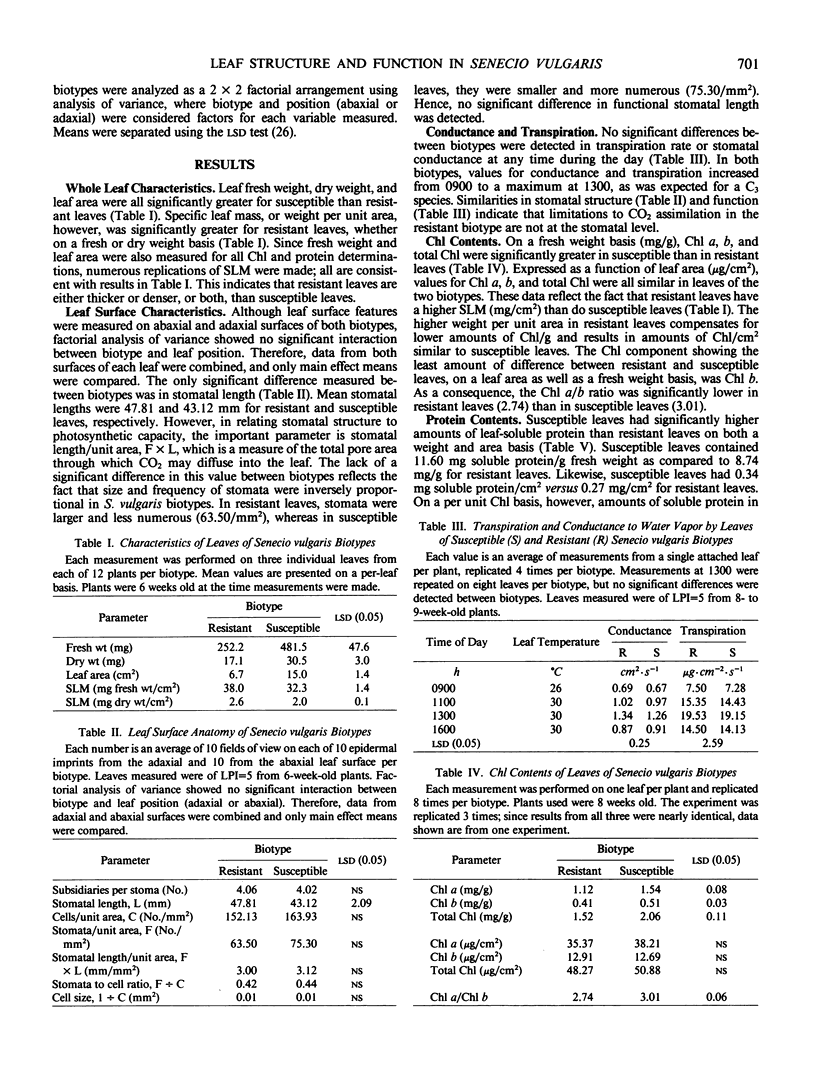
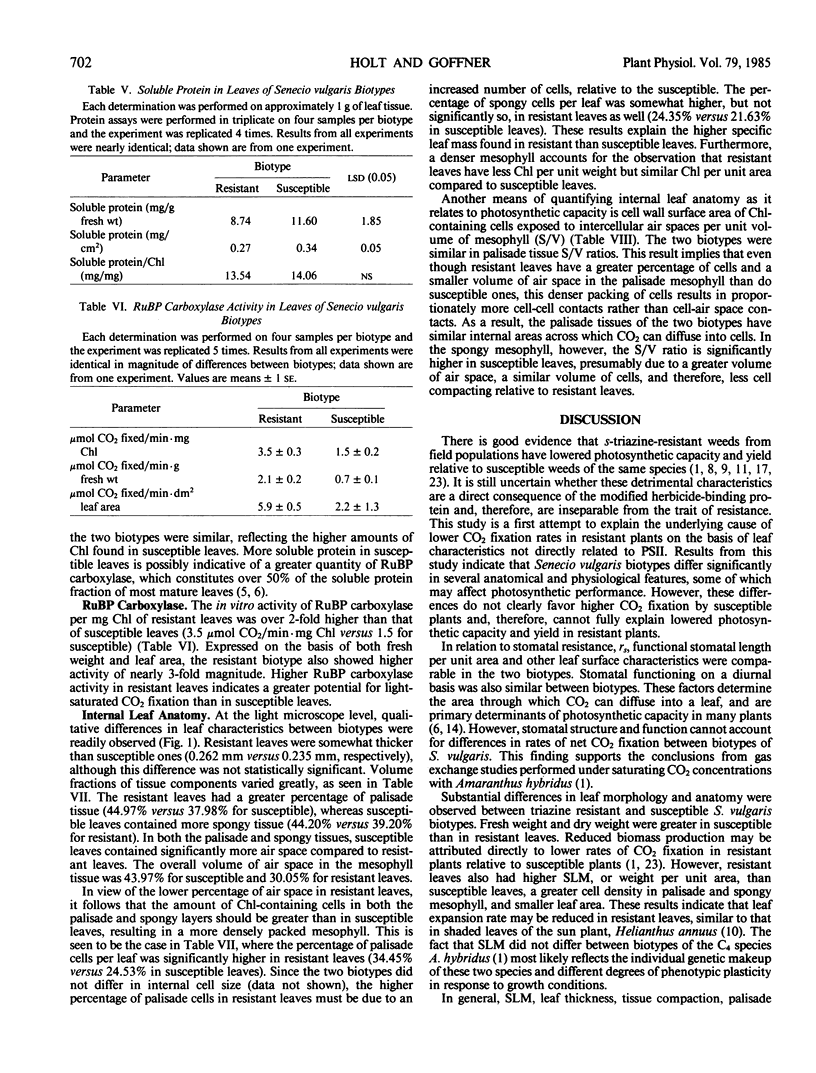
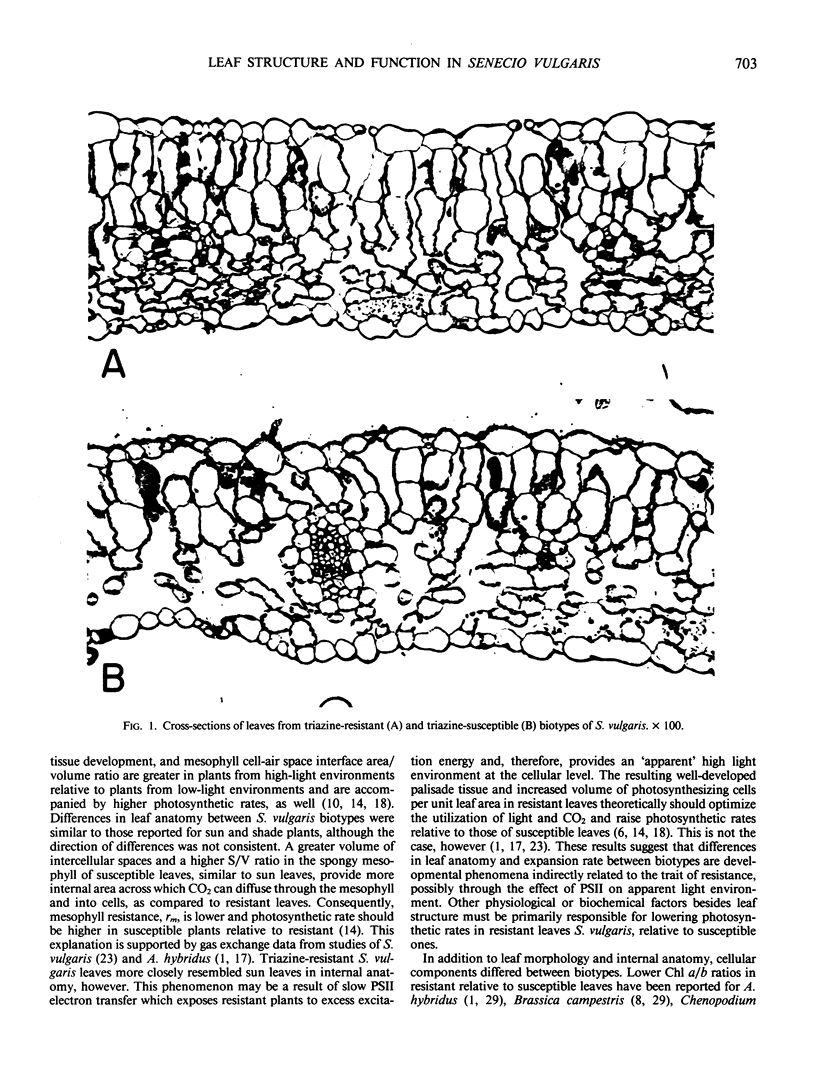
Anatomical and physiological characteristics of leaves of triazinesusceptible and -resistant biotypes of common groundsel (Senecio vulgaris L.) were studied in order to explain the differences in light-saturated photosynthetic rates previously reported. Leaves were of uniform leaf plastochron index from greenhouse-grown plants. Susceptible plants had greater leaf fresh and dry weights and leaf areas, while resistant plants had greater specific leaf mass (mg fresh weight/cm2). Susceptible plants had greater amounts of total chlorophyll per unit leaf weight and a higher chlorophyll a/b ratio. Soluble protein in leaves was higher in susceptible chloroplasts on a weight and area basis, but similar to resistant chloroplasts on a unit chlorophyll basis. Activity of ribulose 1,5-bisphosphate carboxylase was higher in resistant plants on a fresh weight, leaf area, and milligram chlorophyll basis. Stomatal frequency, length, and arrangement were similar between biotypes, as were transpiration and conductance. Resistant leaves had less air space (v/v), more cells in palisade and spongy mesophyll, and a greater volume of palisade tissue than spongy, when compared to susceptible leaves. Differences in leaf structure and function between biotypes are probably due to a complex of developmental adaptations which may be only indirectly related to modified photosystem II in resistant plants. These results indicate that the consistently lower rates of net photosynthesis and yield in resistant plants cannot be explained solely on the basis of these leaf characteristics. Several possible mechanisms to account for reduced productivity are suggested.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A., Andrade S. G., Susin M. Pathological changes due to massive schistosomal infection in man (a case presentation). Rev Inst Med Trop Sao Paulo. 1974 May-Jun;16(3):171–177. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Ditto C. L., Brewer P. E. Chloroplast membrane alterations in triazine-resistant Amaranthus retroflexus biotypes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):278–282. doi: 10.1073/pnas.76.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes J., Crofts A. R., Arntzen C. J. Redox Reactions on the reducing side of photosystem II in chloroplasts with altered herbicide binding properties. Arch Biochem Biophys. 1980 Apr 1;200(2):303–308. doi: 10.1016/0003-9861(80)90359-8. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Wilson R. F., Swafford J. R. Characterization of Chloroplasts Isolated from Triazine-Susceptible and Triazine-Resistant Biotypes of Brassica campestris L. Plant Physiol. 1982 Jul;70(1):24–29. doi: 10.1104/pp.70.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. S., Stemler A. J., Radosevich S. R. Differential Light Responses of Photosynthesis by Triazine-resistant and Triazine-susceptible Senecio vulgaris Biotypes. Plant Physiol. 1981 Apr;67(4):744–748. doi: 10.1104/pp.67.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Ahrens W. H., Martin B., Stoller E. W. Comparison of Photosynthetic Performance in Triazine-Resistant and Susceptible Biotypes of Amaranthus hybridus. Plant Physiol. 1983 Aug;72(4):925–930. doi: 10.1104/pp.72.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E., Terry N. Limiting Factors in Photosynthesis: V. Photochemical Energy Supply Colimits Photosynthesis at Low Values of Intercellular CO(2) Concentration. Plant Physiol. 1984 May;75(1):82–86. doi: 10.1104/pp.75.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]