Abstract
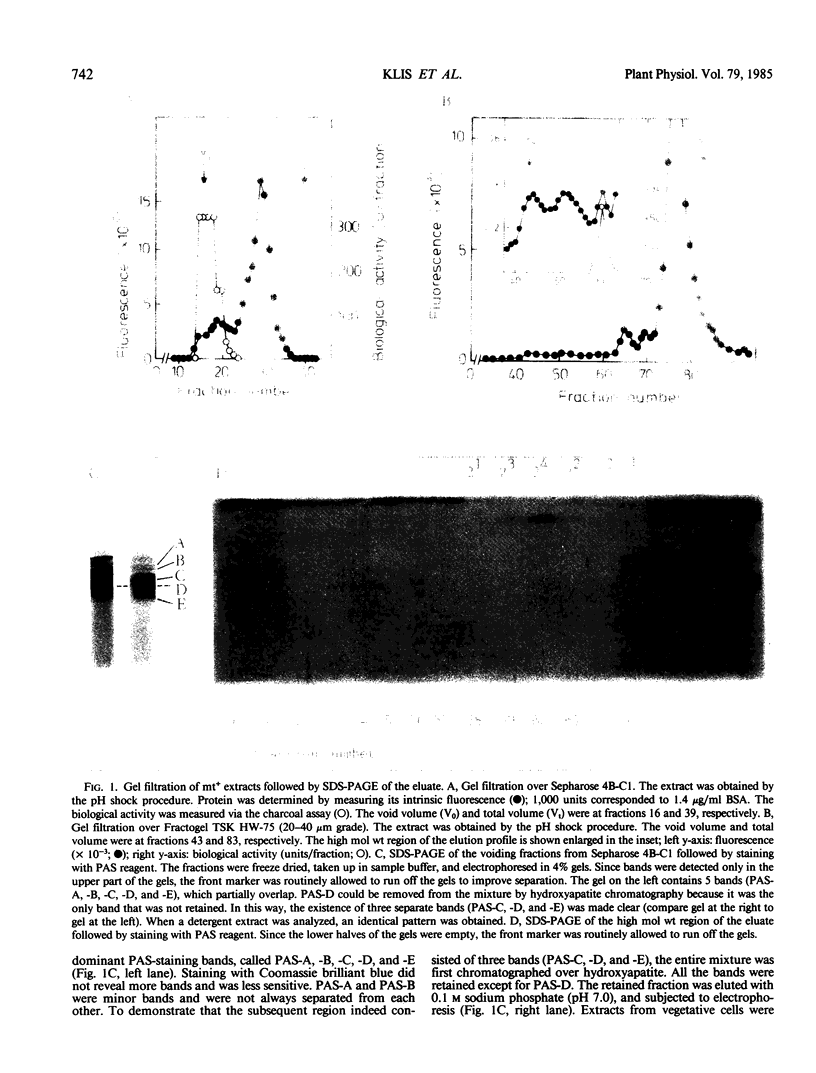
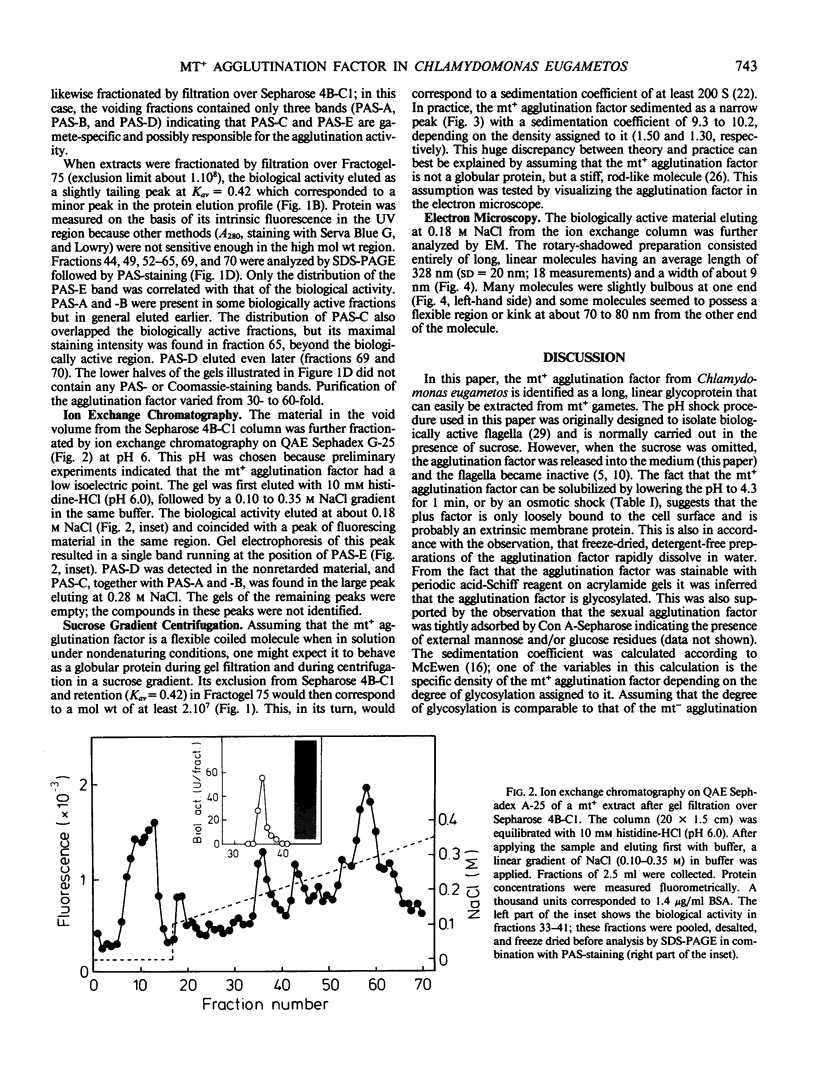
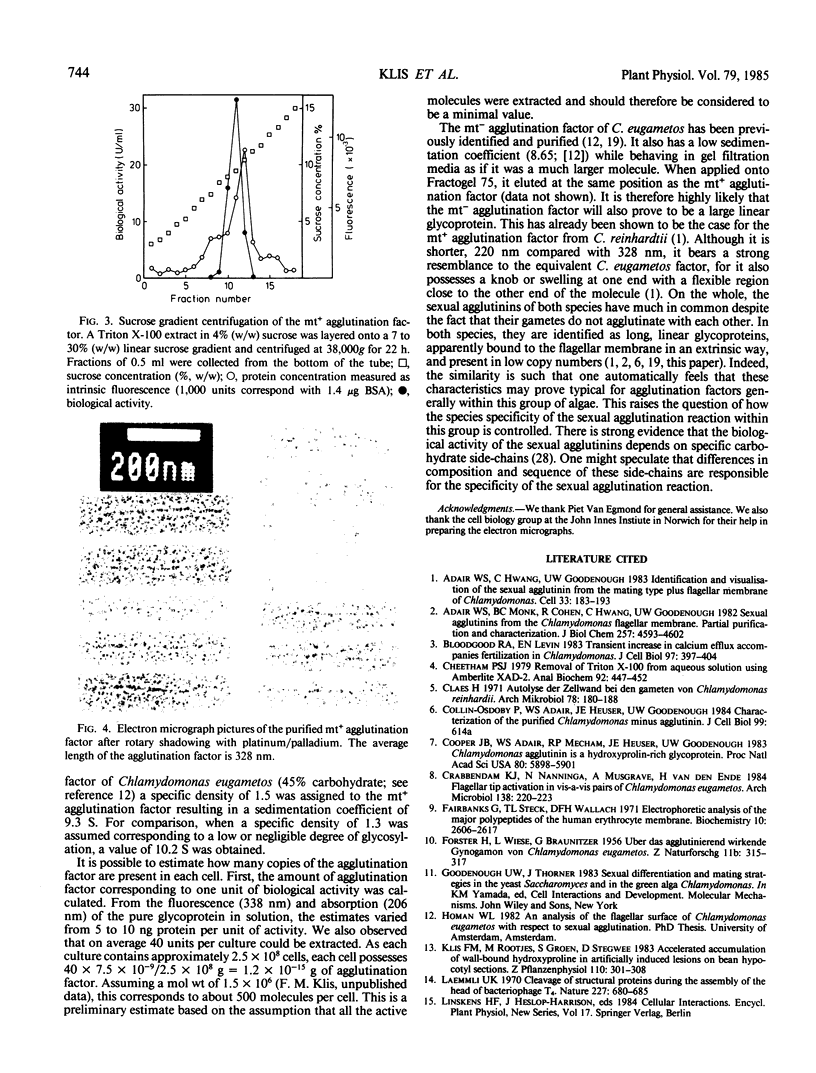
Gametes of the unicellular green alga Chlamydomonas eugametos agglutinate via their flagella. The mating type plus agglutination factor was solubilized by relatively mild treatments such as a short pH shock or an osmotic shock indicating that it is an extrinsic membrane component. It was also extracted in the nonionic detergent Triton X-100. A simple two-step procedure consisting of gel filtration over Sepharose 4B-cross-linked followed by anion exchange chromatography of the void volume yielded an electrophoretically pure preparation of a single high molecular weight glycoprotein. The agglutination factor sedimented as a 9.3 S particle (assuming a density of 1.50) in sucrose gradients. This low value, compared with the high apparent molecular weight seen during gel filtration and electrophoresis, suggests that the agglutination factor is a rod-like molecule. This was confirmed by viewing rotary-shadowed preparations in the electron microscope. A population of long slender molecules was revealed (328 ± 20 nanometers), many of which had a knob at one end and a flexible region about one fourth of the length from the other end.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S., Hwang C., Goodenough U. W. Identification and visualization of the sexual agglutinin from the mating-type plus flagellar membrane of Chlamydomonas. Cell. 1983 May;33(1):183–193. doi: 10.1016/0092-8674(83)90347-1. [DOI] [PubMed] [Google Scholar]
- Adair W. S., Monk B. C., Cohen R., Hwang C., Goodenough U. W. Sexual agglutinins from the Chlamydomonas flagellar membrane. Partial purification and characterization. J Biol Chem. 1982 Apr 25;257(8):4593–4602. [PubMed] [Google Scholar]
- Bloodgood R. A., Levin E. N. Transient increase in calcium efflux accompanies fertilization in Chlamydomonas. J Cell Biol. 1983 Aug;97(2):397–404. doi: 10.1083/jcb.97.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham P. S. Removal of triton X-100 from aqueous solution using amberlite XAD-2. Anal Biochem. 1979 Jan 15;92(2):447–452. doi: 10.1016/0003-2697(79)90683-3. [DOI] [PubMed] [Google Scholar]
- Claes H. Autolyse der Zellwand bei den Gemeten von Chlamydomonas reinhardii. Arch Mikrobiol. 1971;78(2):180–188. [PubMed] [Google Scholar]
- Cooper J. B., Adair W. S., Mecham R. P., Heuser J. E. Chlamydomonas agglutinin is a hydroxyproline-rich glycoprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5898–5901. doi: 10.1073/pnas.80.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Mesland D. A., Hoffman J. L., Caligor E., Goodenough U. W. Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J Cell Biol. 1980 Mar;84(3):599–617. doi: 10.1083/jcb.84.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesland D. A. Mating in Chlamydomonas eugametos. A scanning electron microscopical study. Arch Microbiol. 1976 Aug;109(1-2):31–35. doi: 10.1007/BF00425109. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol. 1954 Jul 20;37(6):729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]