Abstract
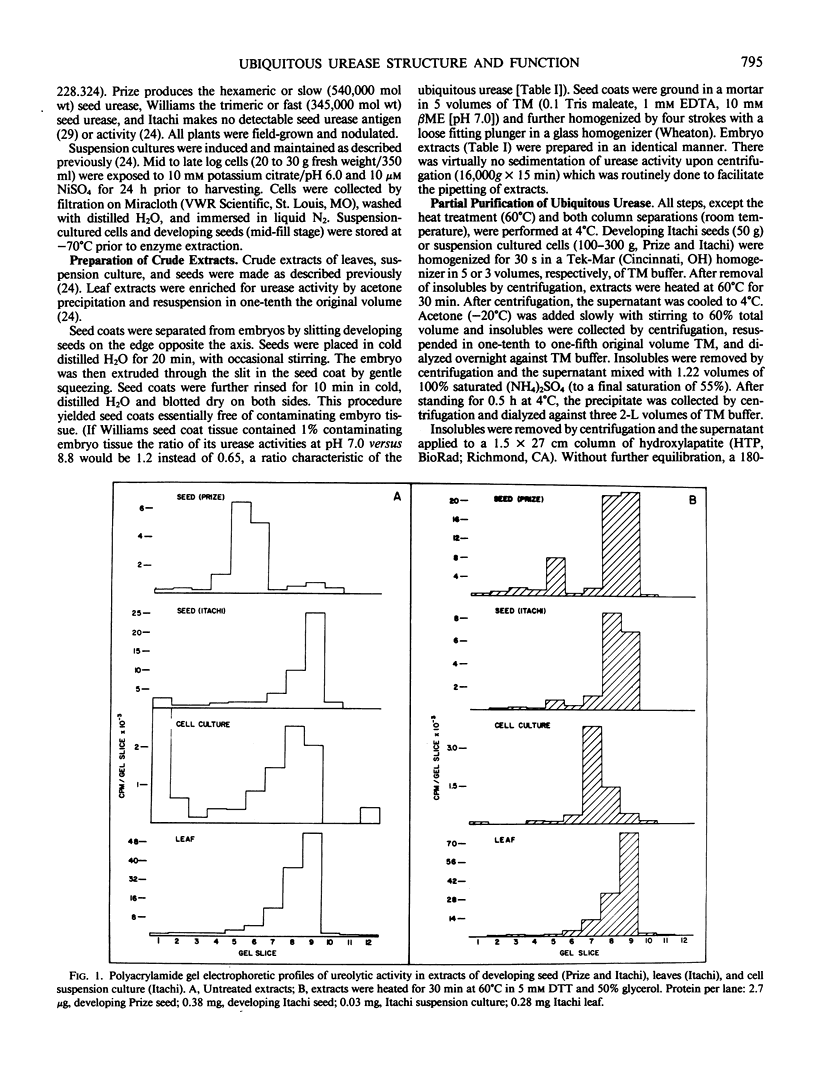
Ubiquitous soybean urease, as opposed to the seed-specific urease, designates the seemingly identical ureolytic activities of suspension cultures and leaves. It also appears to be the basal urease in developing seeds of a variety, Itachi, which lacks the seed-specific urease (Polacco, Winkler 1984 Plant Physiol 74: 800-804). On native polyacrylamide gels the ureolytic activities in crude extracts of these three tissues comigrate as determined by assays of gel slices. At this level of resolution the ubiquitous urease also migrates with or close to the fast (trimeric) form of the seed-specific urease.
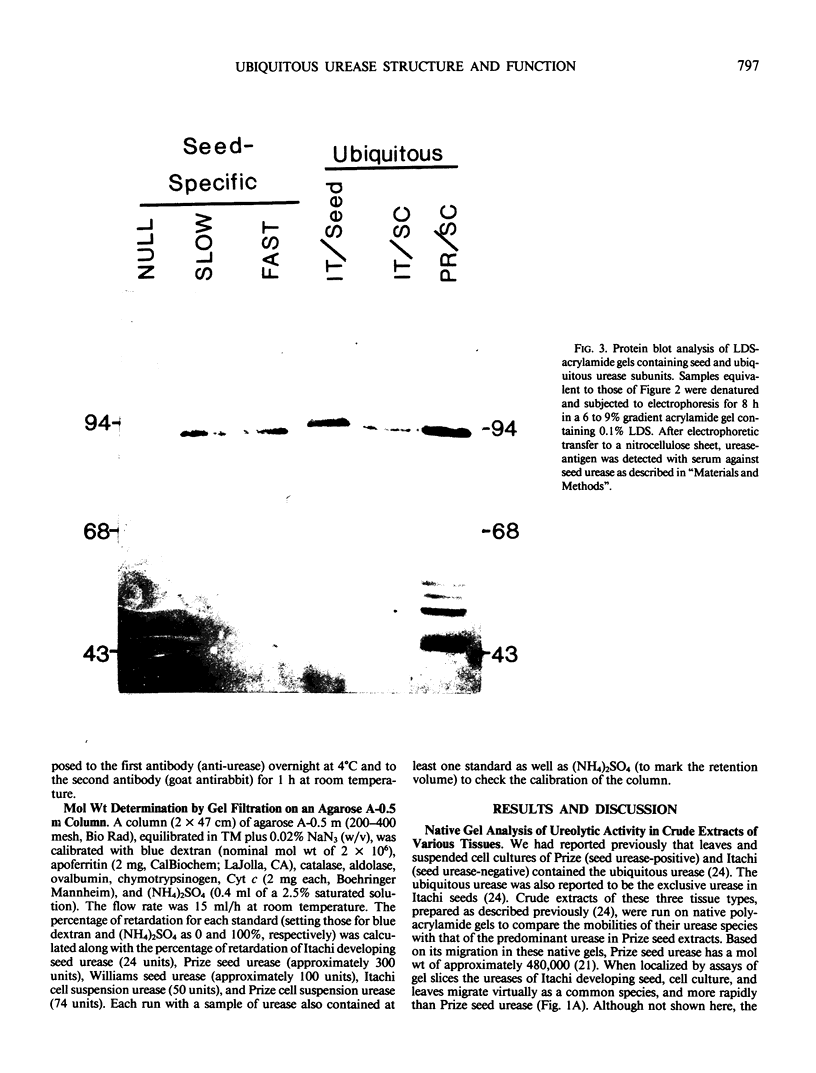
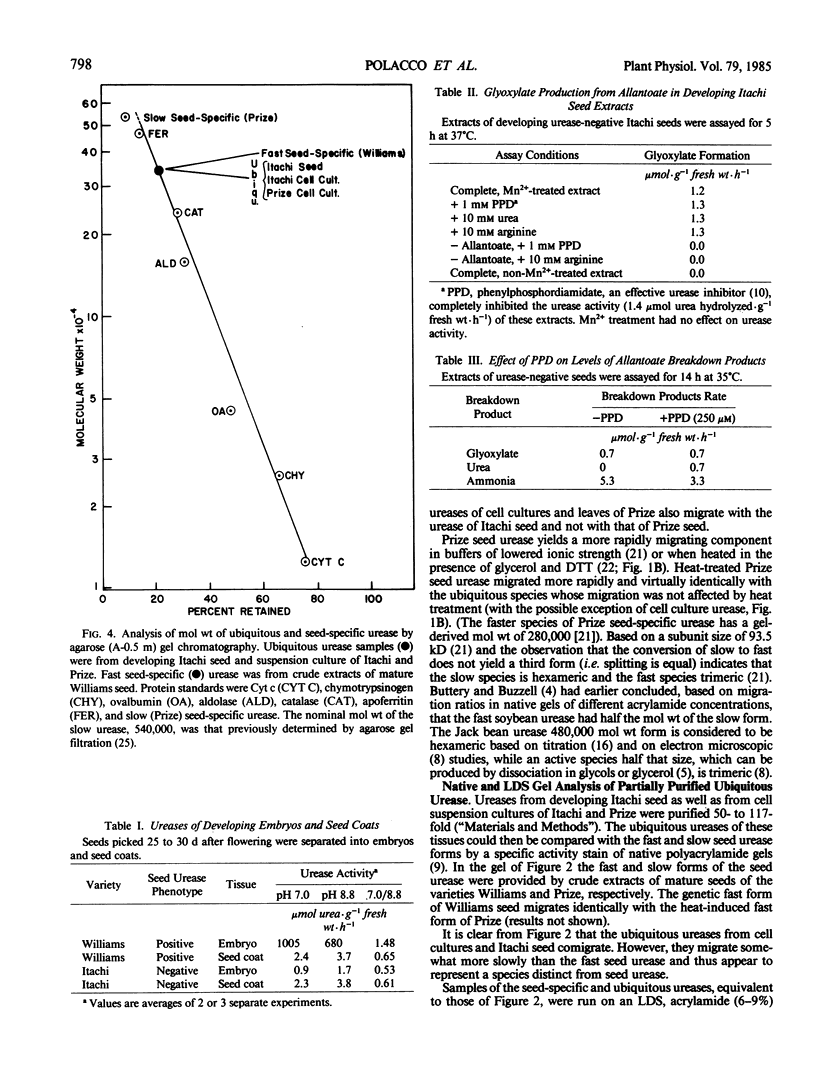
The ubiquitous urease was purified approximately 100-fold from suspension cultures of two cultivars (Itachi and Prize) as well as from developing seeds of Itachi. These partially purified preparations allowed visualization of native urease on polyacrylamide gels by activity staining and of urease subunits on denaturing lithium dodecyl sulfate gels by electrophoretic transfer to nitrocellulose and immunological detection (“Western Blot”). The ubiquitous urease holoenzyme migrates slightly less rapidly than the fast seed urease in native gels; its subunit migrates slightly less rapidly than the 93.5 kilodaltons subunit of either the fast or slow (hexameric) seed enzyme. The ubiquitous urease elutes from an agarose A-0.5 meter column with the fast form of the seed urease species suggesting that the ubiquitous urease, like the fast seed urease, exists as a trimeric holoenzyme. The soybean cultivar, Prize, produces the hexameric seed urease; yet its ubiquitous urease (from leaf and suspension culture) is trimeric.
The pH dependence of the ureolytic activity of seed coats of both seed urease-negative (Itachi) and seed urease-positive (Williams) cultivars suggests that this activity is exclusively the ubiquitous urease. Its relatively higher levels in seed coats than in embryos of Itachi suggests that the ubiquitous urease is involved in degradation of urea derived from ureides. Consistent with a ureide origin for urea is the observation that addition of a urease inhibitor, phenylphosphordiamidate, to extracts of developing Itachi seeds (seed coat plus embryo) results in accumulation of urea from allantoic acid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Ritchie A., Peoples M. B. Metabolism and translocation of allantoin in ureide-producing grain legumes. Plant Physiol. 1982 Aug;70(2):476–482. doi: 10.1104/pp.70.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contaxis C. C., Reithel F. J. Studies on protein multimers. II. A study of the mechanism of urease dissociation in 1,2-propanediol: comparative studies with ethylene glycol and glycerol. J Biol Chem. 1971 Feb 10;246(3):677–685. [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. Nickel: an essential micronutrient for legumes and possibly all higher plants. Science. 1983 Nov 11;222(4624):621–623. doi: 10.1126/science.222.4624.621. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N., Engler W. F., Griffin J. L., Scurzl W., Bahr G. F. Electron microscopy of negatively stained jackbean urease at three levels of quaternary structure, and comparison with hydrodynamic studies. Eur J Biochem. 1977 Feb 15;73(1):185–190. doi: 10.1111/j.1432-1033.1977.tb11306.x. [DOI] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R., Brocklehurst K. A convenient method of preparation of high-activity urease from Canavalia ensiformis by covalent chromatography and an investigation of its thiol groups with 2,2'-dipyridyl disulphide as a thiol titrant and reactivity probe. Biochem J. 1976 Nov;159(2):245–257. doi: 10.1042/bj1590245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Havir E. A. Comparisons of soybean urease isolated from seed and tissue culture. J Biol Chem. 1979 Mar 10;254(5):1707–1715. [PubMed] [Google Scholar]
- Polacco J. C. Nitrogen Metabolism in Soybean Tissue Culture: II. Urea Utilization and Urease Synthesis Require Ni. Plant Physiol. 1977 May;59(5):827–830. doi: 10.1104/pp.59.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C. Nitrogen metabolism in soybean tissue culture: I. Assimilation of urea. Plant Physiol. 1976 Sep;58(3):350–357. doi: 10.1104/pp.58.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Sparks R. B. Patterns of urease synthesis in developing soybeans. Plant Physiol. 1982 Jul;70(1):189–194. doi: 10.1104/pp.70.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Thomas A. L., Bledsoe P. J. A soybean seed urease-null produces urease in cell culture. Plant Physiol. 1982 May;69(5):1233–1240. doi: 10.1104/pp.69.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Winkler R. G. Soybean leaf urease: a seed enzyme? Plant Physiol. 1984 Apr;74(4):800–803. doi: 10.1104/pp.74.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGSON D., HIRAHARA K. The measurement of ammonia in whole blood, erythrocytes, and plasma. J Lab Clin Med. 1957 Jun;49(6):962–974. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Winkler R. G., Polacco J. C., Blevins D. G., Randall D. D. Enzymic degradation of allantoate in developing soybeans. Plant Physiol. 1985 Nov;79(3):787–793. doi: 10.1104/pp.79.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler R. G., Polacco J. C., Eskew D. L., Welch R. M. Nickel is not required for apourease synthesis in soybean seeds. Plant Physiol. 1983 May;72(1):262–263. doi: 10.1104/pp.72.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter H., Kollöffel C. Arginine catabolism in the cotyledons of developing and germinating pea seeds. Plant Physiol. 1983 Nov;73(3):525–528. doi: 10.1104/pp.73.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]