Abstract
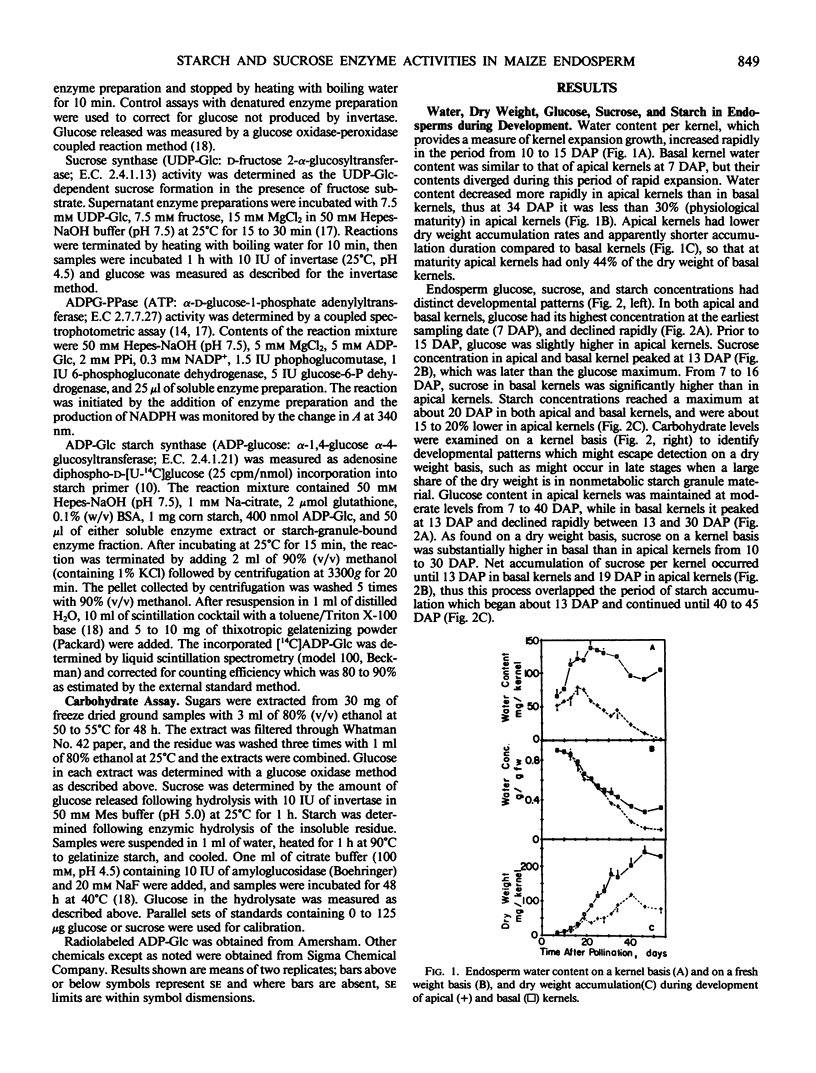
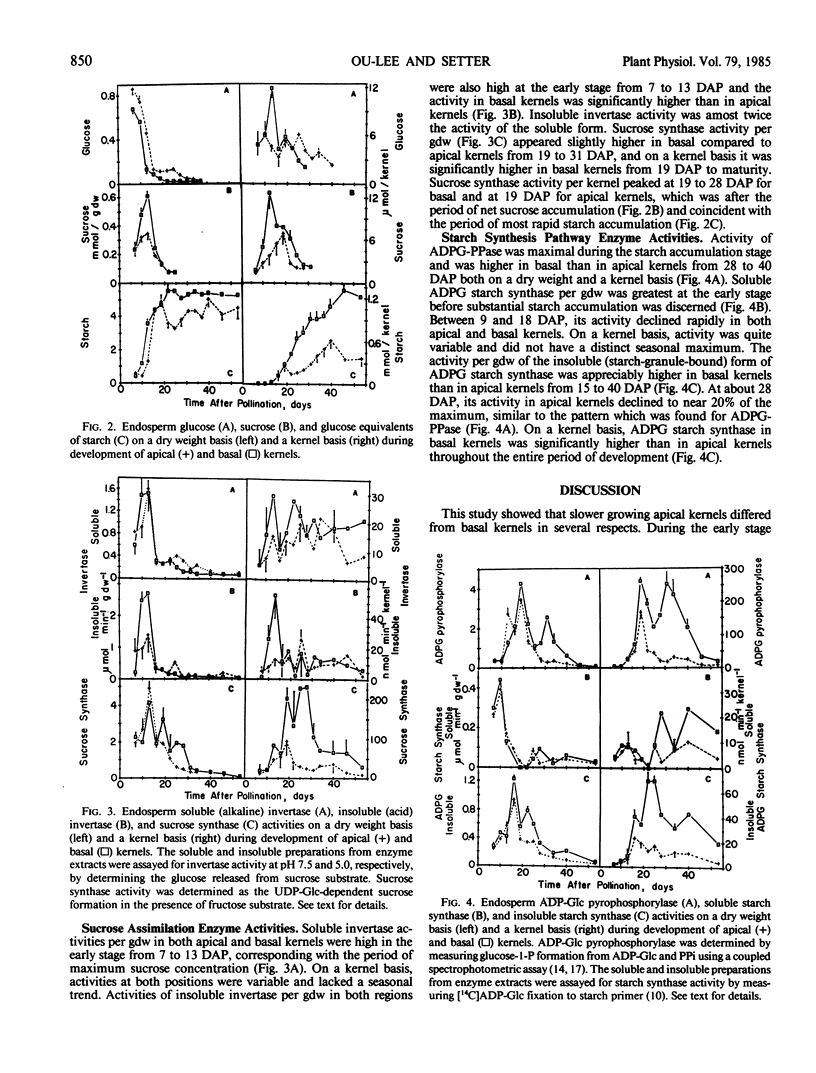
Apical kernels of maize (Zea mays L.) ears have smaller size and lower growth rates than basal kernels. To improve our understanding of this difference, the developmental patterns of starch-synthesis-pathway enzyme activities and accumulation of sugars and starch was determined in apical- and basal-kernel endosperm of greenhouse-grown maize (cultivar Cornell 175) plants. Plants were synchronously pollinated, kernels were sampled from apical and basal ear positions throughout kernel development, and enzyme activities were measured in crude preparations. Several factors were correlated with the higher dry matter accumulation rate and larger mature kernel size of basal-kernel endosperm. During the period of cell expansion (7 to 19 days after pollination), the activity of insoluble (acid) invertase and sucose concentration in endosperm of basal kernels exceeded that in apical kernels. Soluble (alkaline) invertase was also high during this stage but was the same in endosperm of basal and apical kernels, while glucose concentration was higher in apical-kernel endosperm. During the period of maximal starch synthesis, the activities of sucrose synthase, ADP-Glc-pyrophosphorylase, and insoluble (granule-bound) ADP-Glc-starch synthase were higher in endosperm of basal than apical kernels. Soluble ADP-Glc-starch synthase, which was maximal during the early stage before starch accumulated, was the same in endosperm from apical and basal kernels. It appeared that differences in metabolic potential between apical and basal kernels were established at an early stage in kernel development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chourey P. S., Nelson O. E. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet. 1976 Dec;14(11-12):1041–1055. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys. 1969 Mar;130(1):119–128. doi: 10.1016/0003-9861(69)90017-4. [DOI] [PubMed] [Google Scholar]
- Hannah L. C., Nelson O. E. Characterization of adenosine diphosphate glucose pyrophosphorylases from developing maize seeds. Plant Physiol. 1975 Feb;55(2):297–302. doi: 10.1104/pp.55.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. An invertase inactivator in maize endosperm and factors affecting inactivation. Plant Physiol. 1971 May;47(5):629–634. doi: 10.1104/pp.47.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Preiss J. Solubilization of the starch-granule-bound starch synthase of normal maize kernels. Plant Physiol. 1983 Sep;73(1):175–178. doi: 10.1104/pp.73.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Greenberg E., Lammel C., Preiss J. Starch Synthetase, Phosphorylase, ADPglucose Pyrophosphorylase, and UDPglucose Pyrophosphorylase in Developing Maize Kernels. Plant Physiol. 1973 Jan;51(1):1–5. doi: 10.1104/pp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock C., Preiss J. The citrate-stimulated starch synthase of starchy maize kernels: purification and properties. Arch Biochem Biophys. 1980 Oct 15;204(2):578–588. doi: 10.1016/0003-9861(80)90070-3. [DOI] [PubMed] [Google Scholar]
- Rijven A. H. Use of polyethylene glycol in isolation and assay of stable, enzymically active starch granules from developing wheat endosperms. Plant Physiol. 1984 Jun;75(2):323–328. doi: 10.1104/pp.75.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Meller V. H. Reserve carbohydrate in maize stem : [C]glucose and [C]sucrose uptake characteristics. Plant Physiol. 1984 Jul;75(3):617–622. doi: 10.1104/pp.75.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: I. Pattern and Rate of Sugar Movement. Plant Physiol. 1972 Feb;49(2):198–202. doi: 10.1104/pp.49.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: II. Invertase Activity of the Pedicel and Placento-Chalazal Tissues. Plant Physiol. 1972 Feb;49(2):203–206. doi: 10.1104/pp.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. Y., Salamini F., Nelson O. E. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970 Aug;46(2):299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


