Abstract
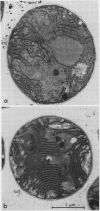
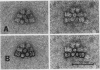
Phycobilisomes isolated from Microcystis aeruginosa grown to midlog at high light (270 microeinsteins per square meter per second) or at low light intensities (40 microeinsteins per square meter per second) were found to be identical. Electron micrographs established that they have a triangular central core apparently consisting of three allophycocyanin trimers surrounded by six rods, each composed of two hexameric phycocyanin molecules. The apparent mass of a phycobilisome obtained by gel filtration is 2.96 × 106 daltons. The molar ratio of the phycobiliproteins per phycobilisome is 12 phycocyanin hexamers:9 allophycocyanin trimers. The electron microscopic observations combined with the phycobilisome apparent mass and the phycobiliprotein stoichiometry data indicate that M. aeruginosa phycobilisomes are composed of a triangular central core of three stacks of three allophycocyanin trimers and six rods each containing two phycocyanin hexamers. Adaptation of M. aeruginosa to high light intensity results in a decrease in the number of phycobilisomes per cell with no alteration in phycobilisome composition or structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diner B. A. Energy Transfer from the Phycobilisomes to Photosystem II Reaction Centers in Wild Type Cyanidium caldarium. Plant Physiol. 1979 Jan;63(1):30–34. doi: 10.1104/pp.63.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Comparative biochemistry of photosynthetic light-harvesting systems. Annu Rev Biochem. 1983;52:125–157. doi: 10.1146/annurev.bi.52.070183.001013. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Williams R. C., Yamanaka G., Schachman H. K. Characterization of cyanobacterial phycobilisomes in zwitterionic detergents. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6162–6166. doi: 10.1073/pnas.76.12.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysi J., Zuber H. Isolation and characterization of allophycocyanin II from the thermophilic blue-green alga Mastigocladus laminosus Cohn. FEBS Lett. 1974 Nov 15;48(2):209–213. doi: 10.1016/0014-5793(74)80469-2. [DOI] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Efficiency of energy transfer from photosystem II to photosystem I in Porphyridium cruentum. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3957–3960. doi: 10.1073/pnas.73.11.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manodori A., Melis A. Photochemical Apparatus Organization in Anacystis nidulans (Cyanophyceae) : Effect of CO(2) Concentration during Cell Growth. Plant Physiol. 1984 Jan;74(1):67–71. doi: 10.1104/pp.74.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Raps S., Wyman K., Siegelman H. W., Falkowski P. G. Adaptation of the Cyanobacterium Microcystis aeruginosa to Light Intensity. Plant Physiol. 1983 Jul;72(3):829–832. doi: 10.1104/pp.72.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Kycia J. H. Molecular morphology of cyanobacterial phycobilisomes. Plant Physiol. 1982 Sep;70(3):887–897. doi: 10.1104/pp.70.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Troxler R. F., Greenwald L. S., Zilinskas B. A. Allophycocyanin from Nostoc sp. phycobilisomes. Properties and amino acid sequence at the NH2 terminus of the alpha and beta subunits of allophycocyanins I, II, and III. J Biol Chem. 1980 Oct 10;255(19):9380–9387. [PubMed] [Google Scholar]