Abstract
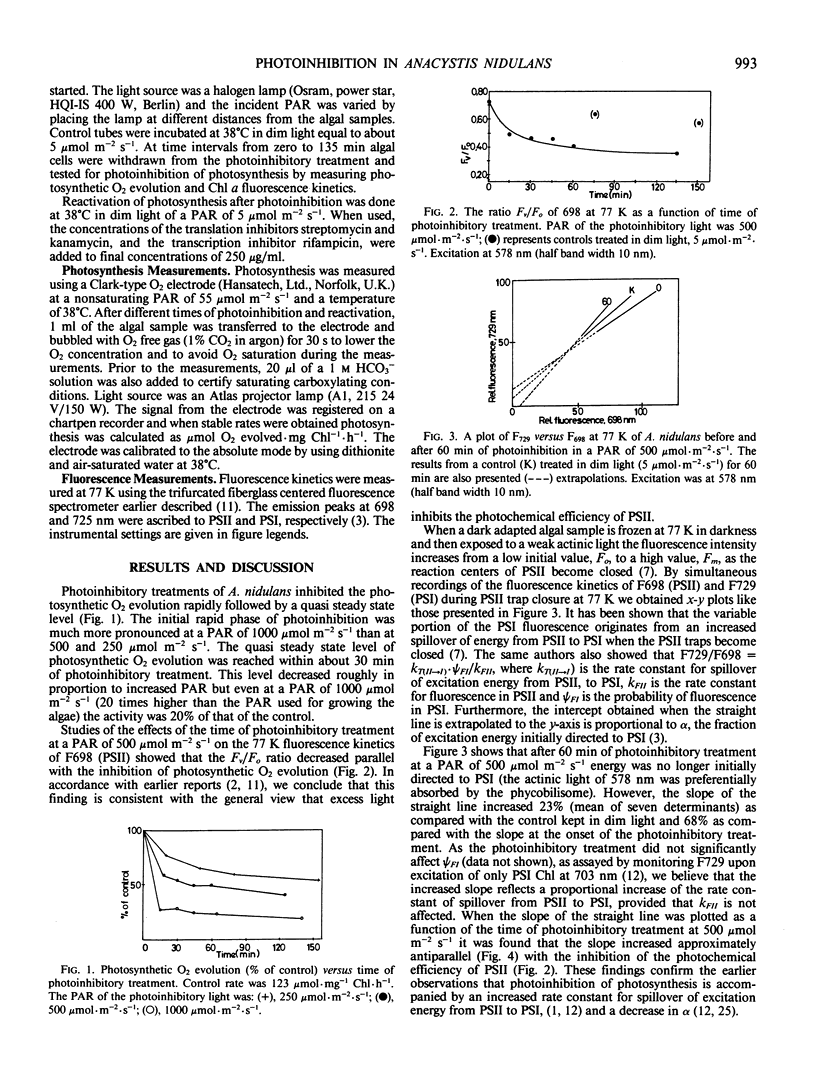
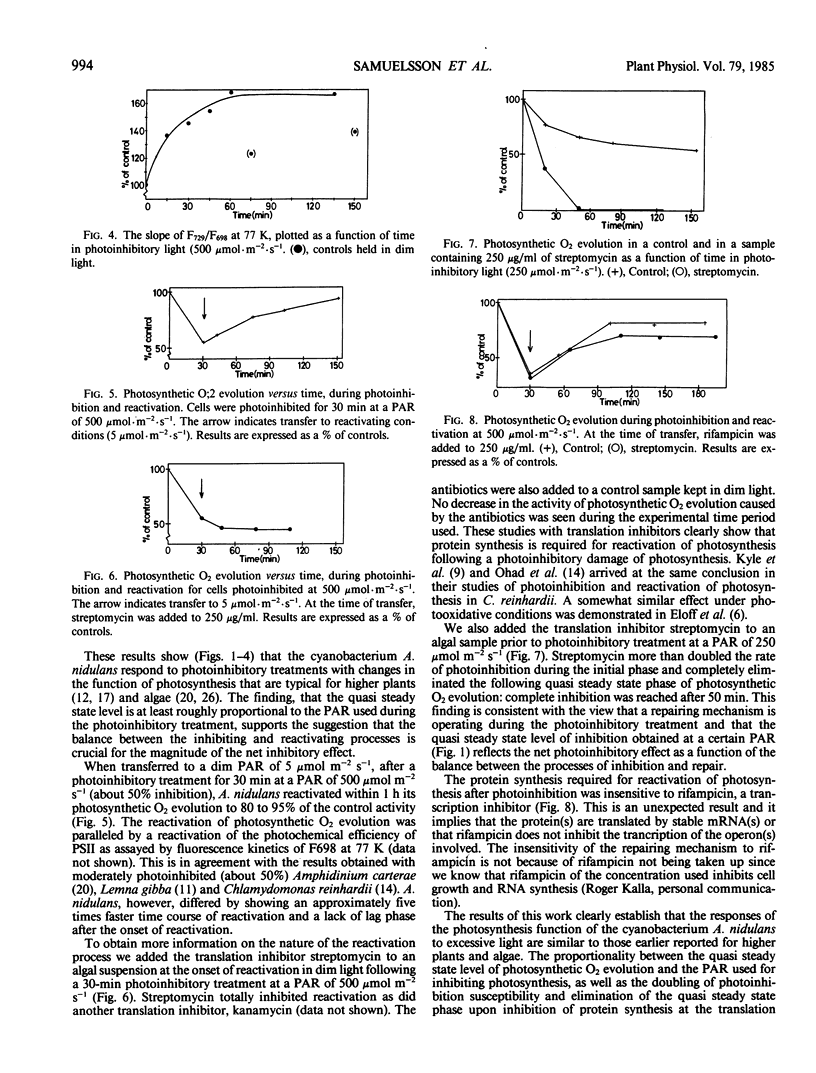
The susceptibility of photosynthesis to photoinhibition and its recovery were studied on cultures of the cyanobacterium Anacystis nidulans. Oxygen evolution and low temperature fluorescence kinetics were measured. Upon exposure to high light A. nidulans showed a rapid decrease in oxygen evolution followed by a quasi steady state rate of photosynthesis. This quasi steady state rate decreased with increasing photon flux density of the photoinhibitory light. Reactivation of photosynthesis in dim light after the photoinhibitory treatment was rapid: 85 to 95% recovery occurred within 2 hours. In the presence of the translation inhibitor, streptomycin (250 micrograms per milliliter), no reactivation occurred. We also found that the damage increased dramatically if the high light treatment was done with streptomycin added. A transcription inhibitor, rifampicin, did not inhibit the reactivation process. Based on these data we conclude that the photoinhibitory damage observed is the net result of a balance between the photoinhibitory process and the operation of the repairing mechanism(s).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Excitation spectra for photosystem I and photosystem II in chloroplasts and the spectral characteristics of the distributions of quanta between the two photosystems. Biochim Biophys Acta. 1975 Dec 11;408(3):297–305. doi: 10.1016/0005-2728(75)90131-0. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. S., Brand J. J., Myers J. Cold Shock Syndrome in Anacystis nidulans. Plant Physiol. 1977 May;59(5):965–969. doi: 10.1104/pp.59.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Fork D. C. Photoinhibition of Reaction Centers of Photosystems I and II in Intact Bryopsis Chloroplasts under Anaerobic Conditions. Plant Physiol. 1982 Oct;70(4):1004–1008. doi: 10.1104/pp.70.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]


