Abstract
Spinach leaf chloroplasts isolated in isotonic media (330 millimolar sorbitol, −1.0 megapascals osmotic potential) had optimum rates of photosynthesis when assayed at −1.0 megapascals. When chloroplasts were isolated in hypertonic media (720 millimolar sorbitol, −2.0 megapascals osmotic potential) the optimum osmotic potential for photosynthesis was shifted to −1.8 megapascals and the chloroplasts had higher rates of CO2-dependent O2 evolution than chloroplasts isolated in 330 millimolar sorbitol when both were assayed at high solute concentrations.
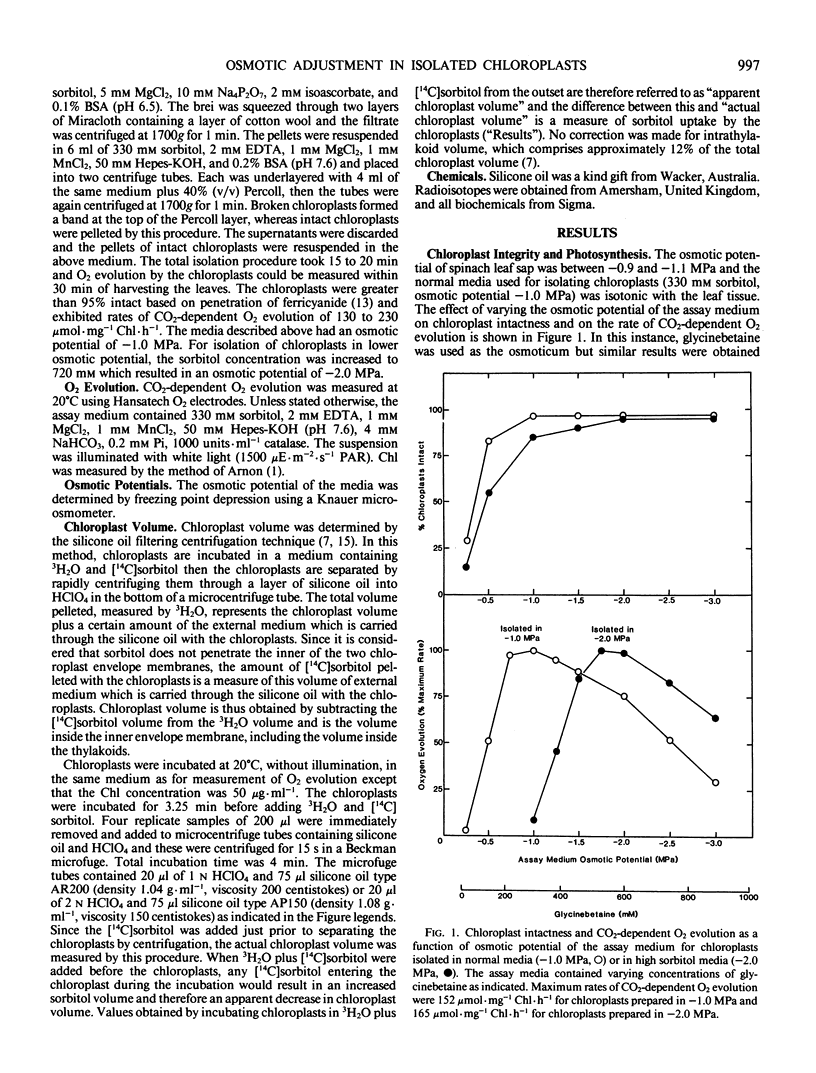
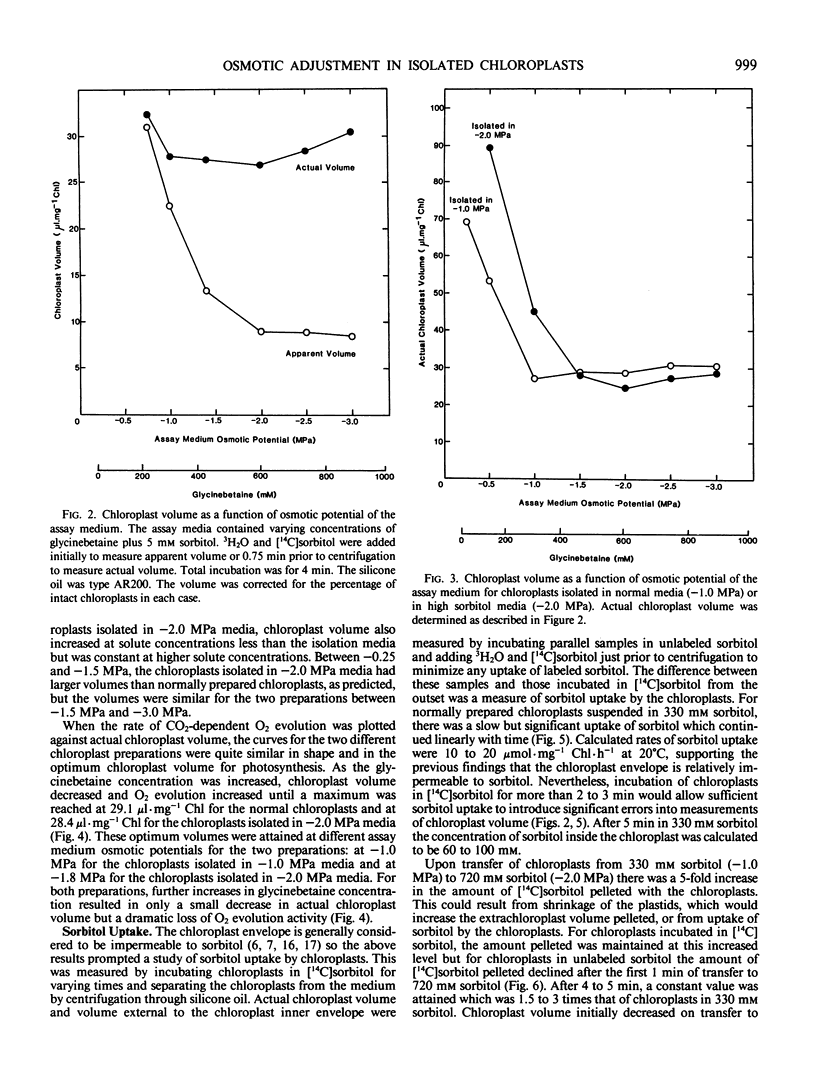
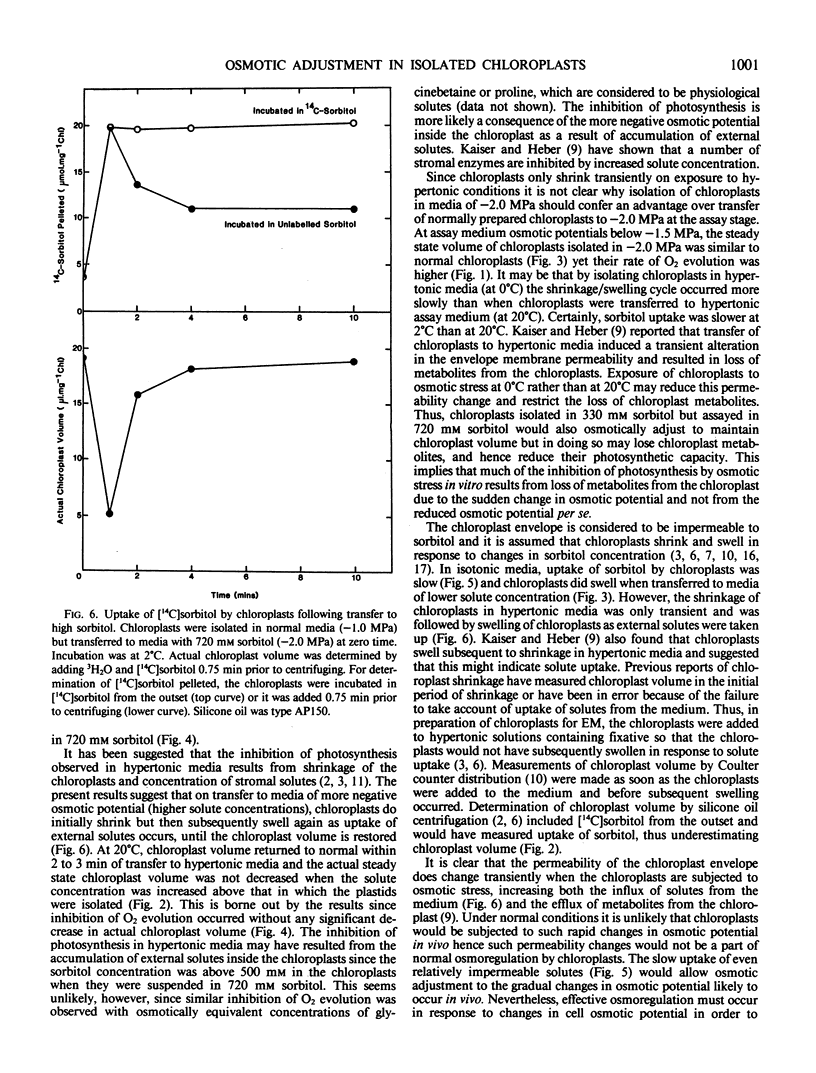
Transfer of chloroplasts isolated in 330 millimolar sorbitol to 720 millimolar sorbitol resulted in decreased chloroplast volume but this shrinkage was only transient and the chloroplasts subsequently swelled so that within 2 to 3 minutes at 20°C the chloroplast volume had returned to near the original value. Thus, actual steady state chloroplast volume was not decreased in hypertonic media. In isotonic media, there was a slow but significant uptake of sorbitol by chloroplasts (10 to 20 micromoles per milligram chlorophyll per hour at 20°C). Transfer of chloroplasts from 330 millimolar sorbitol to 720 millimolar sorbitol resulted in rapid uptake of sorbitol (up to 280 micromoles per milligram chlorophyll per hour at 20°C) and after 5 minutes the concentration of sorbitol inside the chloroplasts exceeded 500 millimolar. This uptake of sorbitol resulted in a significant underestimation of chloroplast volume unless [14C]sorbitol was added just prior to centrifuging the chloroplasts through silicone oil. Sudden exposure to osmotic stress apparently induced a transient change in the permeability of the chloroplast envelope since addition of [14C]sorbitol 3 minutes after transfer to hypertonic media (when chloroplast volume had returned to normal) did not result in rapid uptake of labeled sorbitol.
It is concluded that chloroplasts can osmotically adjust in vitro by uptake of solutes which do not normally penetrate the chloroplast envelope, resulting in a restoration of normal chloroplast volume and partially preventing the inhibition of photosynthesis by high solute concentrations. The results indicate the importance of matching the osmotic potential of isolation media to that of the tissue, particularly in studies of stress physiology.
Full text
PDF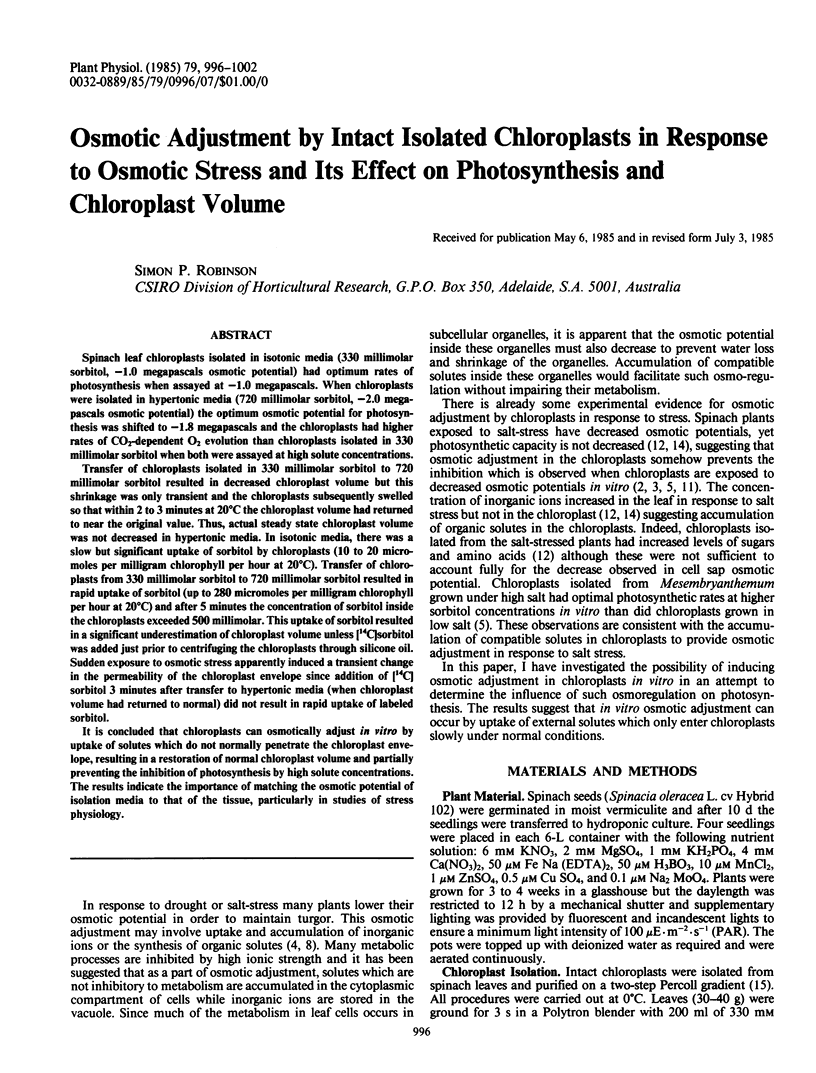



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential inhibition of photosynthesis : site-specific effects of osmotically induced stromal acidification. Plant Physiol. 1983 Aug;72(4):1100–1109. doi: 10.1104/pp.72.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Downton W. J., Millhouse J. A. Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol. 1983 Oct;73(2):238–242. doi: 10.1104/pp.73.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Lack of ATP requirement for light stimulation of glycerate transport into intact isolated chloroplasts. Plant Physiol. 1984 Jun;75(2):425–430. doi: 10.1104/pp.75.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Nobel P. S. Permeability of pea chloroplasts to alcohols and aldoses as measured by reflection coefficients. Biochim Biophys Acta. 1971 Jul 6;241(1):200–212. doi: 10.1016/0005-2736(71)90317-8. [DOI] [PubMed] [Google Scholar]


