Abstract
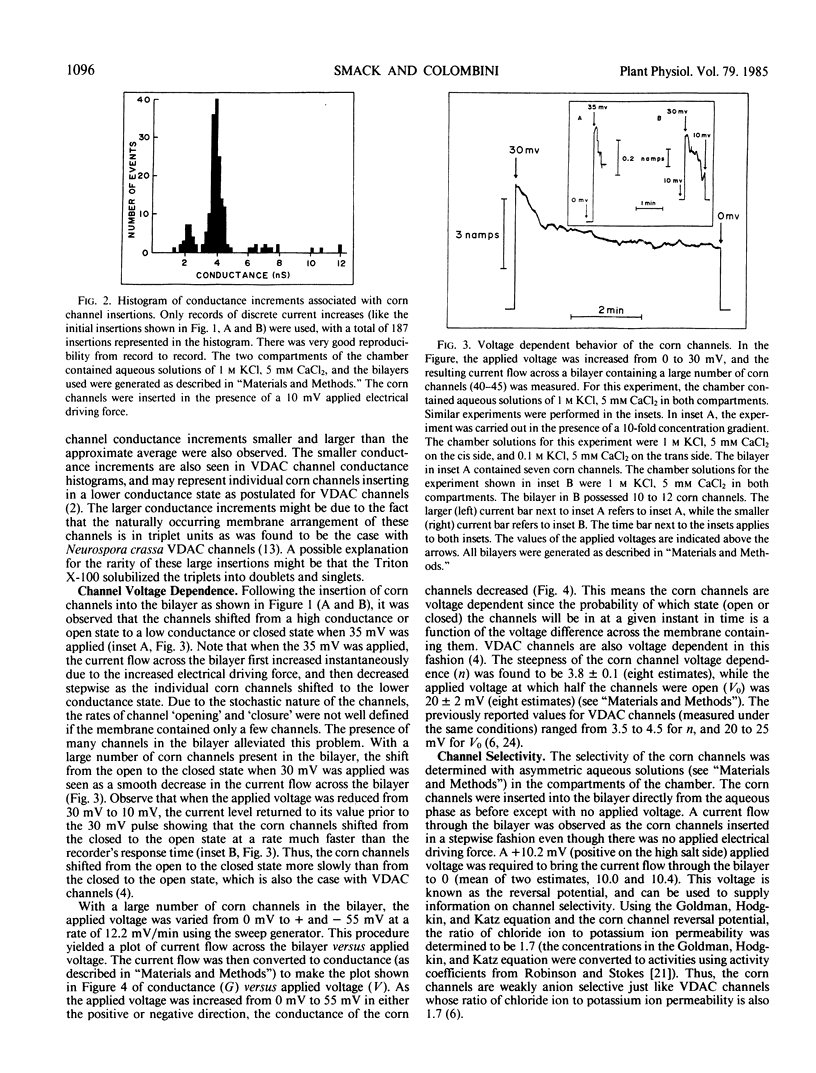
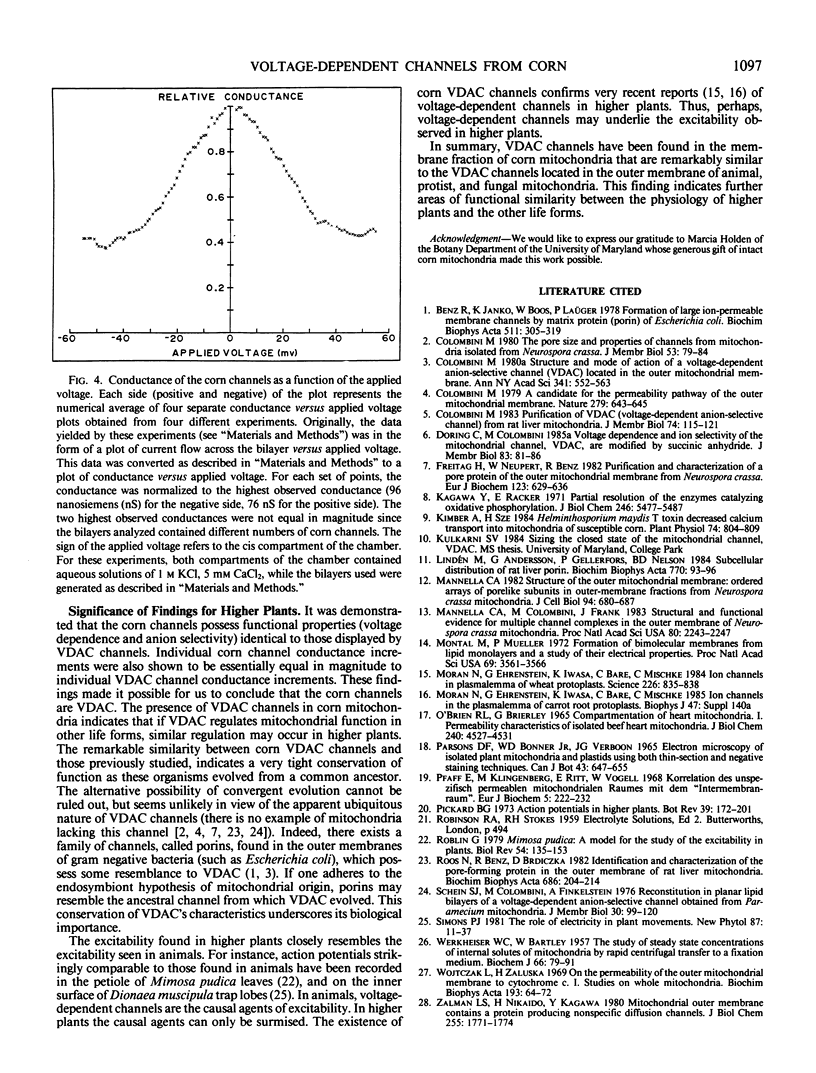
Transmembrane channels have been found in the membrane fraction of corn (Zea mays W64AN) mitochondria that exhibit a remarkable resemblance to the voltage dependent anion-selective channels (VDAC) located in the outer membrane of animal (Rattus norvegicus), protist (Paramecium aurelia), and fungal (Neurospora crassa) mitochondria. The channels in corn were demonstrated to be essentially identical to VDAC channels in three characteristic properties: (a) single channel conductance magnitude, (b) weak anion selectivity, and (c) nature of voltage dependence. These findings led us to conclude that the channels present in corn mitochondria are VDAC channels. This discovery may have repercussions concerning the regulation and function of higher plant mitochondria, and the causation of higher plant excitability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M. Purification of VDAC (voltage-dependent anion-selective channel) from rat liver mitochondria. J Membr Biol. 1983;74(2):115–121. doi: 10.1007/BF01870500. [DOI] [PubMed] [Google Scholar]
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- Doring C., Colombini M. Voltage dependence and ion selectivity of the mitochondrial channel, VDAC, are modified by succinic anhydride. J Membr Biol. 1985;83(1-2):81–86. doi: 10.1007/BF01868740. [DOI] [PubMed] [Google Scholar]
- Freitag H., Neupert W., Benz R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur J Biochem. 1982 Apr;123(3):629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Kimber A., Sze H. Helminthosporium maydis T Toxin Decreased Calcium Transport into Mitochondria of Susceptible Corn. Plant Physiol. 1984 Apr;74(4):804–809. doi: 10.1104/pp.74.4.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Andersson G., Gellerfors P., Nelson B. D. Subcellular distribution of rat liver porin. Biochim Biophys Acta. 1984 Feb 29;770(1):93–96. doi: 10.1016/0005-2736(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Colombini M., Frank J. Structural and functional evidence for multiple channel complexes in the outer membrane of Neurospora crassa mitochondria. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2243–2247. doi: 10.1073/pnas.80.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982 Sep;94(3):680–687. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Brierley G. Compartmentation of heart mitochondria. I. Permeability characteristics of isolated beef heart mitochondria. J Biol Chem. 1965 Nov;240(11):4527–4531. [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Ritt E., Vogell W. Korrelation des unspezifisch permeablen mitochondrialen Raumes mit dem "Intermembran-Raum". Eur J Biochem. 1968 Jul;5(2):222–232. doi: 10.1111/j.1432-1033.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Roos N., Benz R., Brdiczka D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim Biophys Acta. 1982 Apr 7;686(2):204–214. doi: 10.1016/0005-2736(82)90114-6. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Zaluska H. On the impermeability of the outer mitochondrial membrane to cytochrome c. I. Studies on whole mitochondria. Biochim Biophys Acta. 1969 Oct 14;193(1):64–72. doi: 10.1016/0005-2736(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Nikaido H., Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980 Mar 10;255(5):1771–1774. [PubMed] [Google Scholar]


