Abstract
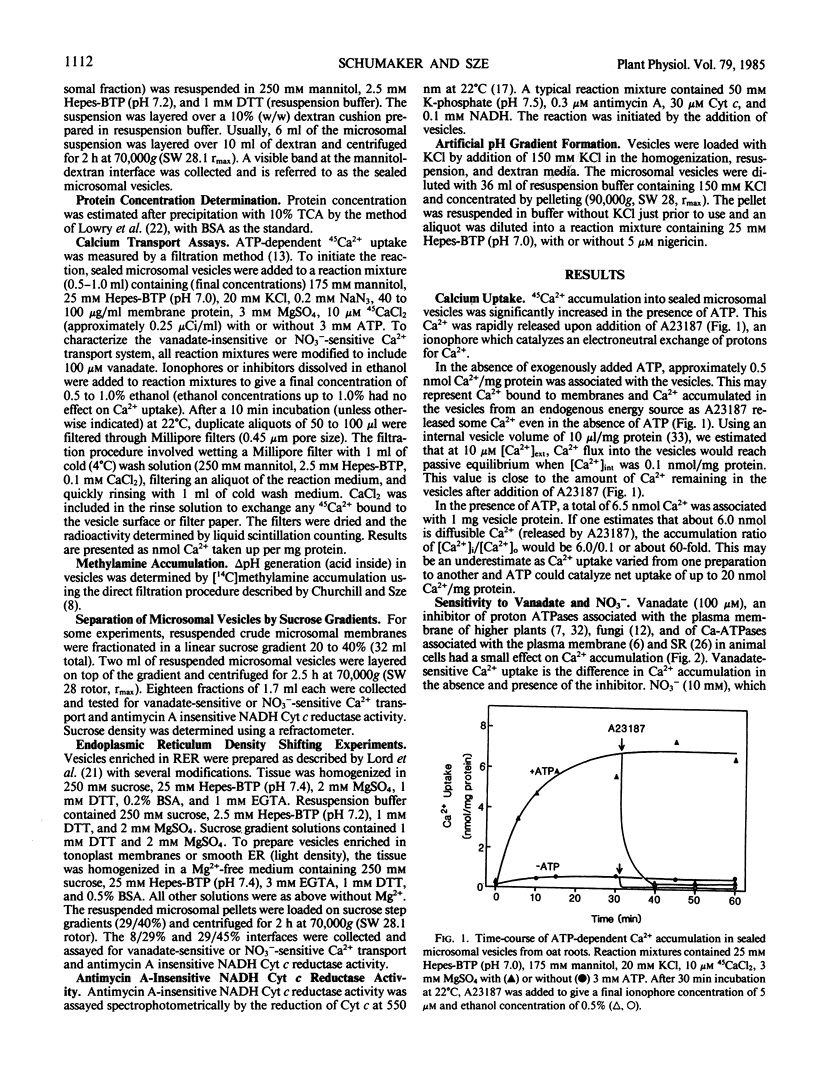
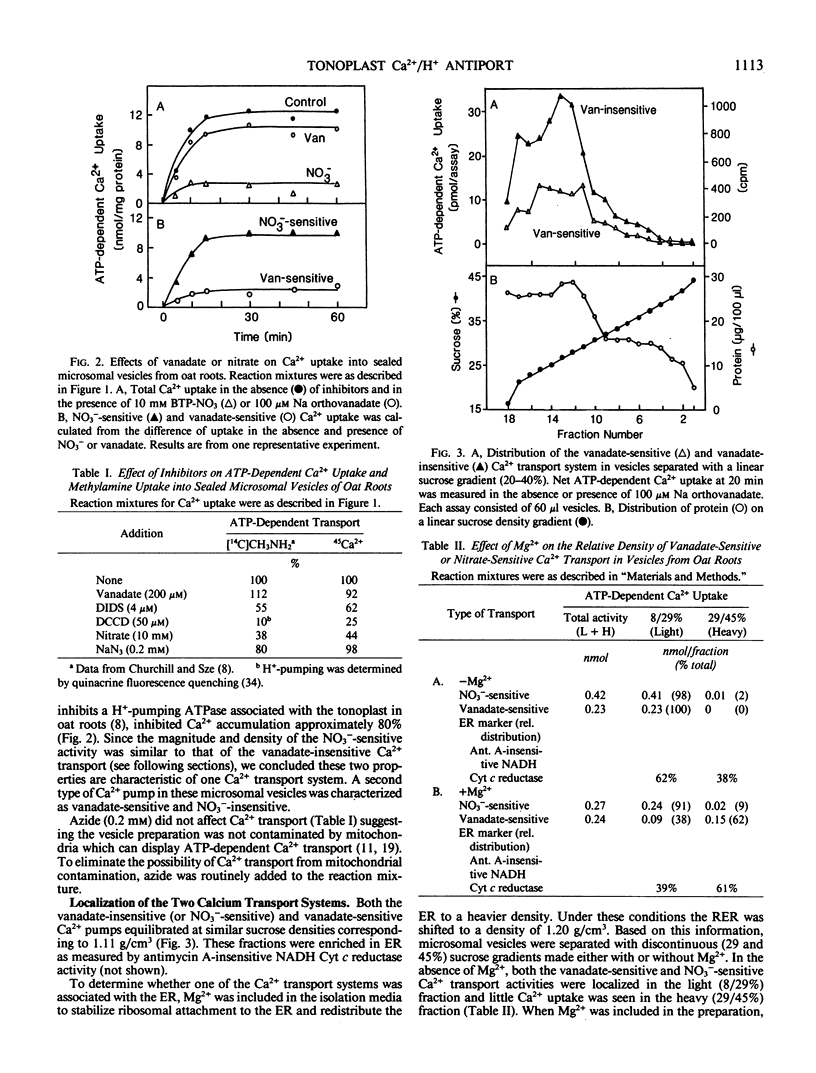
Two types of ATP-dependent calcium (Ca2+) transport systems were detected in sealed microsomal vesicles from oat roots. Approximately 80% of the total Ca2+ uptake was associated with vesicles of 1.11 grams per cubic centimeter and was insensitive to vanadate or azide, but inhibited by NO3−. The remaining 20% was vanadate-sensitive and mostly associated with the endoplasmic reticulum, as the transport activity comigrated with an endoplasmic reticulum marker (antimycin A-insensitive NADH cytochrome c reductase), which was shifted from 1.11 to 1.20 grams per cubic centimeter by Mg2+.
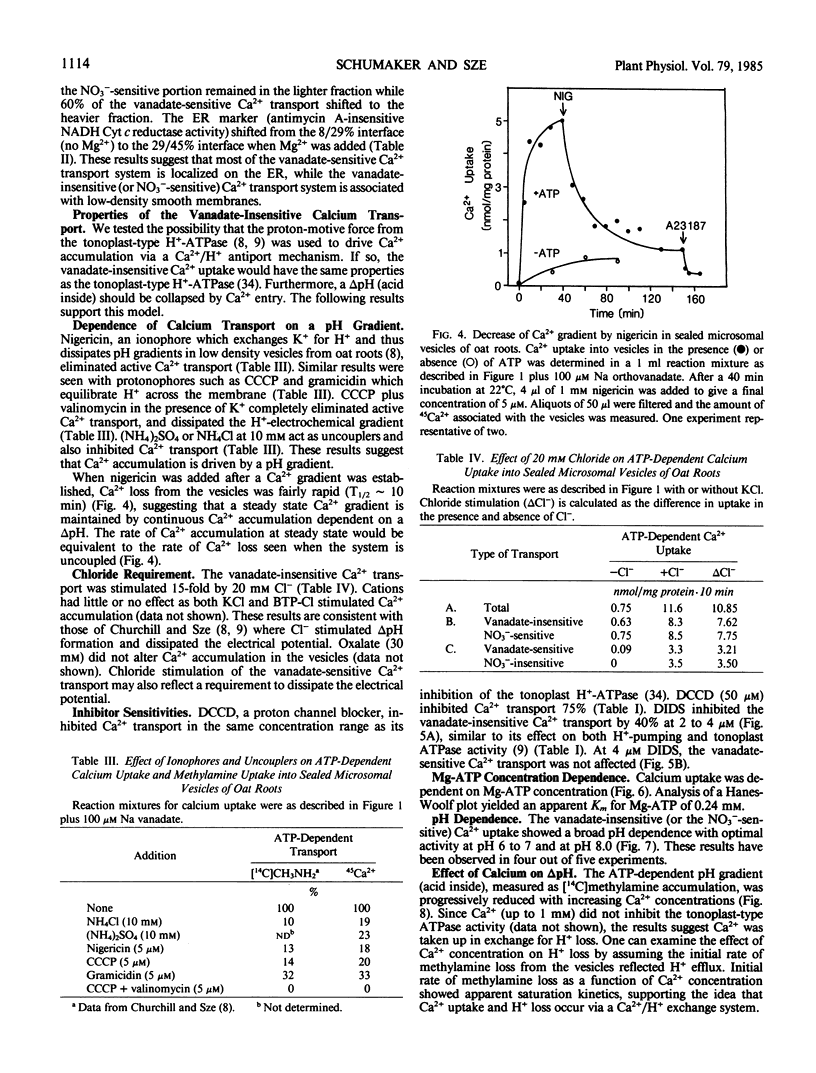
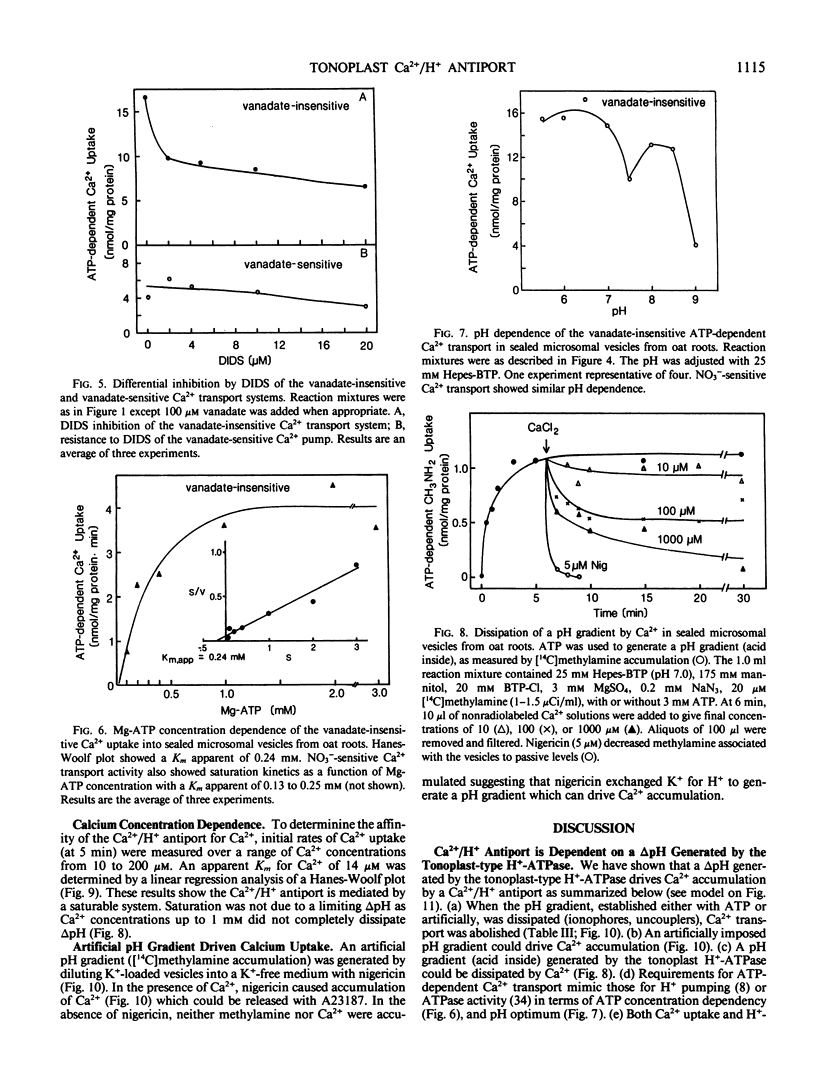
Like the tonoplast H+-ATPase activity, vanadate-insensitive Ca2+ accumulation was stimulated by 20 millimolar Cl− and inhibited by 10 micromolar 4,4′-diisothiocyano-2,2′-stilbene disulfonic acid or 50 micromolar N,N′-dicyclohexylcarbodiimide. This Ca2+ transport system had an apparent Km for Mg-ATP of 0.24 millimolar similar to the tonoplast ATPase. The vanadate-insensitive Ca2+ transport was abolished by compounds that eliminated a pH gradient and Ca2+ dissipated a pH gradient (acid inside) generated by the tonoplast-type H+-ATPase. These results provide compelling evidence that a pH gradient generated by the H+-ATPase drives Ca2+ accumulation into right-side-out tonoplast vesicles via a Ca2+/H+ antiport. This transport system was saturable with respect to Ca2+ (Km apparent = 14 micromolar). The Ca2+/H+ antiport operated independently of the H+-ATPase since an artifically imposed pH gradient (acid inside) could also drive Ca2+ accumulation. Ca2+ transport by this system may be one major way in which vacuoles function in Ca2+ homeostasis in the cytoplasm of plant cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borle A. B. Control, Modulation, and regulation of cell calcium. Rev Physiol Biochem Pharmacol. 1981;90:13–153. doi: 10.1007/BFb0034078. [DOI] [PubMed] [Google Scholar]
- Brey R. N., Rosen B. P. Cation/proton antiport systems in Escherichia coli. Properties of the calcium/proton antiporter. J Biol Chem. 1979 Mar 25;254(6):1957–1963. [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-Sensitive, H-Pumping ATPase of Oat Roots : Direct Effects of Cl, NO(3), and a Disulfonic Stilbene. Plant Physiol. 1984 Oct;76(2):490–497. doi: 10.1104/pp.76.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K. Membranes in the mitotic apparatus of barley cells. J Cell Biol. 1980 Aug;86(2):490–499. doi: 10.1083/jcb.86.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Inesi G. Mechanism of calcium transport. Annu Rev Physiol. 1985;47:573–601. doi: 10.1146/annurev.ph.47.030185.003041. [DOI] [PubMed] [Google Scholar]
- Kimber A., Sze H. Helminthosporium maydis T Toxin Decreased Calcium Transport into Mitochondria of Susceptible Corn. Plant Physiol. 1984 Apr;74(4):804–809. doi: 10.1104/pp.74.4.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983 May 10;258(9):5614–5617. [PubMed] [Google Scholar]
- Pick U. The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J Biol Chem. 1982 Jun 10;257(11):6111–6119. [PubMed] [Google Scholar]
- Stroobant P., Scarborough G. A. Active transport of calcium in Neurospora plasma membrane vesicles. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3102–3106. doi: 10.1073/pnas.76.7.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sze H. Similarities and differences between the tonoplast-type and the mitochondrial H+-ATPases of oat roots. J Biol Chem. 1985 Sep 5;260(19):10434–10443. [PubMed] [Google Scholar]
- Williamson R. E., Ashley C. C. Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature. 1982 Apr 15;296(5858):647–650. doi: 10.1038/296647a0. [DOI] [PubMed] [Google Scholar]


