Abstract
A complex metabolic condition referred to as Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance (IR) and decreased insulin production. Obesity, dyslipidemia, hypertension, and chronic inflammation are just a few of the cardiometabolic illnesses that people with T2DM are more likely to acquire and results in cardiovascular issues. It is essential to comprehend the mechanistic insights into these risk variables in order to prevent and manage cardiovascular problems in T2DM effectively. Impaired glycemic control leads to upregulation of De novo lipogenesis (DNL), promote hepatic triglyceride (TG) synthesis, worsening dyslipidemia that is accompanied by low levels of high density lipoprotein cholesterol (HDL-C) and high amounts of small, dense low-density lipoprotein cholesterol (LDL-C) further developing atherosclerosis. By causing endothelial dysfunction, oxidative stress, and chronic inflammation, chronic hyperglycemia worsens already existing cardiometabolic risk factors. Vasoconstriction, inflammation, and platelet aggregation are caused by endothelial dysfunction, which is characterized by decreased nitric oxide production, increased release of vasoconstrictors, proinflammatory cytokines, and adhesion molecules. The loop of IR and endothelial dysfunction is sustained by chronic inflammation fueled by inflammatory mediators produced in adipose tissue. Infiltrating inflammatory cells exacerbate inflammation and the development of plaque in the artery wall. In addition, the combination of chronic inflammation, dyslipidemia, and IR contributes to the emergence of hypertension, a prevalent comorbidity in T2DM. The ability to target therapies and management techniques is made possible by improvements in our knowledge of these mechanistic insights. Aim of present review is to enhance our current understanding of the mechanistic insights into the cardiometabolic risk factors related to T2DM provides important details into the interaction of pathophysiological processes resulting in cardiovascular problems. Understanding these pathways will enable us to create efficient plans for the prevention, detection, and treatment of cardiovascular problems in T2DM patients, ultimately leading to better overall health outcomes.
Keywords: Type 2 diabetic mellitus (T2DM), cardiometabolic risk factors, obesity, dyslipidemia, hypertension, diabetic nephropathy, cardiovascular diseases, mortality
Plain Language Summary
Understanding the factors that increase the risk of type 2 diabetes: Exploring how the body works
Type 2 diabetes mellitus (T2DM) is a complex condition where the body struggles to use insulin properly and doesn’t produce enough of it. This often leads to other health issues like obesity, high cholesterol, high blood pressure, and chronic inflammation. These problems increase the risk of heart and blood vessel diseases in people with T2DM. To tackle these issues effectively, it’s crucial to understand the underlying mechanisms. When blood sugar levels are not controlled, the body starts making more fat and storing it in the liver, leading to high triglycerides and low levels of good cholesterol. This process can block arteries, causing heart problems. High blood sugar also damages blood vessel linings, making them inflamed and less functional. This inflammation, combined with other factors like high cholesterol and insulin resistance, can lead to high blood pressure. Chronic inflammation, where the body’s defense system stays active for too long, worsens these problems. In T2DM, inflammation occurs in fat tissues, making the situation even worse. Inflammatory cells infiltrate blood vessel walls, promoting plaque buildup and further worsening heart issues. Understanding these processes helps us develop better strategies to prevent, detect, and treat heart problems in people with T2DM. By targeting these mechanisms, doctors can create more effective plans to improve the overall health of individuals with diabetes and reduce the risk of cardiovascular diseases.
Introduction
As the world’s most prevalent cause of mortality, Non communicable diseases (NCDs) are considered to pose a serious threat to the public health worldwide. Other than cancer, cardiovascular diseases and diabetes are gaining international attention as the prime NCDs affecting global population. 1 Cardiovascular diseases are attributed for 31% of global deaths by world health organization in 2019 and its prevalence is rapidly expanding as a significant global health concern. 2 On the other hand, according to international diabetes federation (IDF) in 2021, 8.5% of adult global population is suffering from diabetes, with an estimate of 643 million cases by 2045. 3 Among the 2 distinct forms of diabetes that is, type 1 and Type 2, Type 2 diabetes mellitus (T2DM) accounts for approximately 95% of global diabetes cases, diagnosed maximally in individuals over 40 years of age. 4 In the realm of modern medicine, the intricate interplay of genetic predispositions, environmental factors, and complex physiological mechanisms underlying Type 2 Diabetes Mellitus (T2DM) has captivated the attention of researchers and clinicians alike. 5 T2DM patients have a 2 to 5 times higher risk of developing macrovascular disease than non-diabetic individual as glycemia is a persistent contributing factor for development of vascular complication with no discernible threshold. Furthermore, as 60% to 70% of patient with diabetes is clinically obese, 70% to 80% are suffering from hypertension or have high LDL-C levels, the variables of cardiovascular risks plays a vital counterpart for overall health of an individual. 6 The focus of recent research has shifted from merely identifying cardiometabolic risk factors associated with T2DM to deciphering the intricate molecular pathways governing these connections. This evolution in our comprehension brings forth a fundamental question: how can these intricate mechanistic insights are translated into pragmatic clinical practices? This inquiry is not just an intellectual pursuit; it represents a critical bridge between scientific discovery and tangible improvements in patient outcomes. In this context, this exploration delves into the pivotal role of translating these mechanistic revelations into the real-world context of clinical care. In this review the prime objective of the authors is to identify the cardiovascular comorbidities associated with T2DM and highlight the underlying interconnected mechanisms of vascular dysfunction that elucidate cardiovascular outcomes in T2DM.
This review focus in the necessity of managing the metabolic complications in T2DM patients to prevent the occurrence of serious cardiovascular outcome. Also the therapeutic targets and clinical interventions highlighted give an overviewing insight for clinical practitioners, the importance to assay and check cardiometabolic risk factors along with glycemic management, that the authors believe will act as a base for designing a combination intervention therapy, minimizing mortality in T2DM patients due to CVD.
Overview of cardiometabolic risk factor
Cardiometabolic risk factors are a group of condition that raises the possibility of metabolic diseases like T2DM and several CVD. The predominant cardiometabolic factors that enhance the probability of CVD occurrence include hypertension, dyslipidemia, obesity, IR, impaired glucose tolerance and unhealthy lifestyle. 7
A significant cardiometabolic risk factor, obesity is characterized by an excessive buildup of body fat. It is linked to IR, adipose tissue malfunction, and a chronic low-grade inflammatory state by releasing several pro-inflammatory cytokines that majorly includes tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-12, IL-1b, Elastases, cathepsin G, and Proteinase-3. 8 Another significant component of cardiometabolic risk is IR that alludes to decreased cellular receptivity to insulin’s effects impairing glucose uptake and utilization along with promoting hyperuricemia, hypertension, elevated serum inflammatory markers and also develops a prothombic state. Genetic predispositions, obesity, lack of exercise, and persistent inflammation can all have an impact on IR. 9 As a result, there is compensatory hyperinsulinemia, which worsens the deregulation of lipid metabolism and encourages the growth of atherosclerosis. In dyslipidemia, the bloodstream’s lipid (cholesterol and triglyceride) levels are abnormal. 10 Atherosclerosis, which is characterized by the buildup of plaque in the artery walls, is a condition made worse by dyslipidemia. Atherosclerotic plaques can develop as a result of deposition of cholesterol in the artery intima causing inflammation and impaired blood flow. 11 High blood pressure also known as hypertension is a significant CVD risk factor. Increased peripheral vascular resistance, suboptimal endothelial function and anomalies in the Renin-Angiotensin-Aldosterone System (RAAS) are a few of the processes that it may be brought on by hypertension. Hypertension also encourages vascular damage, speeds up atherosclerosis, and puts more strain on the heart, which all help to cause heart disease. 12 Cardiometabolic risk factors frequently overlap and influence the onset and course of metabolic and CVDs synergistically. A nutritious diet, frequent exercise, and weight control are all essential lifestyle changes that can significantly lower these risk factors.
Understanding diabetes
T2DM involves 2 factors: malfunction in insulin secretion by pancreatic β cells and impaired insulin sensitivity thereby failing to cope up with metabolic necessities. 13 T2DM is a complex amalgamation of genetic, metabolic and environmental risk factors that contributes to the prevalence of the disease. Under healthy physiological state, insulin produced by β-cell is triggered to be released once the blood glucose level is abnormally high. The adenosine triphosphate (ATP) dependent potassium channel and cyclic adenosine monophosphate (cAMP) that balance the intracellular calcium ion (Ca2+) concentration induce insulin secretion.14,15 Several mechanisms have been reveals that are responsible for the dysfunction of pancreatic β cells, thus impairs insulin16,17
Adipose tissue alterations that occur with obesity lead to an increase in the release of free fatty acids into the circulation. The insulin signaling in muscle and liver cells can be disrupted by these raised amounts of FFA, which can result in decreased glucose uptake and increased gluconeogenesis. 18 Anomalies in the insulin receptors themselves can lead to IR as well. These receptors are surface-addressed proteins that bind to insulin to start the signaling chain that leads to glucose absorption. Cells may occasionally become less sensitive to insulin if there are fewer insulin receptors or changes to their structure or function. 19 Numerous intracellular signaling pathways are used by insulin to affect target cells. The phosphoinositide 3-kinase (PI3K)/Akt pathway is one significant route. Defects in this pathway can happen at a number of different levels in IR. Impairment of PI3K activation, lower Akt activity, and disruptions in insulin receptor substrate (IRS) phosphorylation can all result in impaired insulin signaling and IR. Reduced glucose transporter protein type 4 (GLUT4) translocation to the cell membrane is another common cause of IR. In reaction to insulin, GLUT4 promotes glucose uptake into cells. This translocation process can be interfered with by impaired insulin signaling, which lowers the efficiency of glucose uptake by cells, notably muscle and adipose cells.20,21 Blood glucose levels are maintained in part by the liver. The liver promotes gluconeogenesis in T2DM, raising blood sugar levels. Under normal physiologic condition insulin inhibits gluconeogenesis, but during IR gluconeogenesis is boosted in liver. 22
Also the phenomenon of fatty pancreas, characterized by the accumulation of excess fat within the pancreatic tissue, has emerged as a significant player in the realm of insulin resistance and T2DM pathogenesis that not only mirrors peripheral insulin resistance but also contributes to a unique aspect of the disease. A key concept in understanding this intricate relationship is the twin-cycle hypothesis according to which, excess free fatty acids in the bloodstream resulting from obesity and poor dietary choices, lead to both peripheral insulin resistance and an increased influx of fatty acids into the liver. Within the liver, these fatty acids are metabolized, generating lipid metabolites and promoting hepatic insulin resistance. Simultaneously, these lipid metabolites find their way back to the pancreas interfering with the normal βcell function, impairing insulin secretion. This dual hit insulin resistance in peripheral tissues and impaired insulin secretion due to fatty infiltration in the pancreas creates a vicious cycle, further exacerbating the progression of T2DM. Understanding this twin-cycle hypothesis sheds light on the intricate relationship between fatty pancreas and insulin resistance, offering valuable insights for targeted interventions aimed at breaking this detrimental cycle and managing T2DM more effectively. 23
Cardiovascular Complication in Diabetes
T2DM is a leading cause of morbidity and mortality in people with cardiac conditions because it is linked to an increased risk of cardiovascular problems. Diabetes’s chronic hyperglycemia and metabolic abnormalities accelerate the onset and progression of a number of cardiovascular illnesses including coronary artery disease (CAD), myocardial infarction (MI), stroke, peripheral arterial disease (PAD). These diabetic cardiovascular problems are caused by various mechanisms encouraged by chronic hyperglycemia, causing atherosclerotic plaques to develop in artery walls. The usual diabetes complications of IR, dyslipidemia, hypertension, and obesity also raise the risk of CVD.24,25
As compared to non-diabetics, T2DM patients typically experience CAD at an earlier stage, with more rapid progression and with more severe outcomes having pathogenesis that combines vascular, inflammatory, and metabolic alterations.26,27 Endothelial dysfunction occurs early in the course of CAD development. In T2DM, nitric oxide (NO) bioavailability is diminished and reactive oxygen species (ROS) are produced as a result of persistent hyperglycemia and IR resulting in reduced vasodilation, greater vasoconstriction, and enhanced inflammatory cell adherence to artery walls. Endothelial dysfunction hinders the coronary arteries’ capacity to widen in response to an increase in demand and encourages the development of atherosclerotic plaques which is a significant cause of CAD and is promoted by several variables.28,29 Furthermore diabetes is linked to dyslipidemia where increased LDL-C and triglyceride levels encourage the buildup of lipids within the artery walls, which results in the development of atherosclerotic plaque. 30 Chronic low-grade inflammation brought on by elevated levels of pro-inflammatory cytokines including IL-6 and TNF-α in diabetes accelerate atherosclerosis by infiltration of inflammatory cells into the artery walls, such as macrophages and T lymphocytes. 31 Diabetes affects the microvasculature of the heart as well as the big coronary arteries. Blood flow to the myocardium is decreased by microvascular dysfunction characterized by reduced vasodilation and increased vasoconstriction, resulting in MI, 32 also referred to as heart attack that occurs when blood supply to a portion of the heart muscle is blocked, causing tissue damage and cell death. Compared to non-diabetics, T2DM patients have much higher risk of MI occurrence. Vascular, metabolic, and inflammatory alterations are all involved in the pathogenesis of MI in diabetes.33,34 When blood flow to the brain is interrupted the risk of cerebrovascular accident develops, commonly termed as stroke that results in neurological impairments. 35 According to data from the Greater Cincinnati/Northern Kentucky stroke research, T2DM raises the risk of ischemic stroke in all age groups. 36 Diabetes is reported to cause microvascular diseases in which arteries of the brain thickens, this anatomical alteration cause brain tissue damage leading to development of lacunar infarcts. Furthermore, in T2DM patients macrovascular conditions like atherosclerosis, develop ruptured atherosclerotic plaques causes damages to the big arteries that are supplying blood to the brain.37,38 Furthermore, the risk of stroke development in T2DM can also be influenced by thrombosis and embolism in addition to atherosclerosis and inflammatory conditions. 39 T2DM patients are observed develop pro-thrombotic state, altering the coagulation system and raise the possibility of blood clots forming in the cerebral arteries thus in turn increase the risk of ischemic stroke. Additionally, diabetic condition manifests the development of atrial fibrillation (AF), an unstable heart rhythm characterized by irregular and fast contractions of the atria. Blood clots formed in the heart chambers as a result of AF may then move to the brain causing an ischemic stroke.40,41
Atherosclerosis in the lower extremities is known as PAD, and is correlated with atherothrombosis in other vascular beds, such as the cardiovascular and cerebrovascular systems. In comparison to nondiabetic people, T2DM patients have significantly higher chance of developing PAD and an accelerated course of the disease, rendering them more vulnerable to serious cardiovascular events.42,43 The two risk variables with the highest odds ratios for PAD are T2DM and smoking and have odds ratios of 2.72 and 1.88, respectively. 44 However, as smoking rates decline in Western nations, T2DM emerge as the main causative factor in the onset and progression of PAD. An aberrant ankle-brachial index (ABI) is more common in people with glucose intolerance (more than 20% prevalence), compared to 7% in people with normal glucose tolerance. 45 Hyperglycemia, dyslipidemia, and IR in T2DM promotes the development and progression of PAD due to derangements in the vessel wall by upregulation of vascular inflammation and dysfunctioning of endothelial cell; variability in blood cells, including smooth muscle cells and platelets; and factors that affect hemostasis.42,46 C-reactive protein (CRP), a biomarker of inflammation, is linked to both the onset of PAD and poor glycemic control 47 by elevating procoagulant tissue factor, leukocyte adhesion molecules, and chemotactic chemicals. Also endothelial nitric oxide synthase (eNOS), which generates NO via a phosphoinositol-3-kinase dependent route, is inhibited by CRP, resulting in vascular tone abnormalities acting as causative factor of PAD.48,49
Dyslipidemia and Diabetes
Dyslipidemia is characterized by a range of quantitative and qualitative abnormalities in lipids and lipoproteins, is a frequently occurring metabolic disorder linked to T2DM. Hypertriglyceridemia, lowered HDL-C levels, and a shift to small dense LDL-C make up the prevalent pattern of lipid abnormalities known as diabetic dyslipidemia. 50
Lipid abnormalities in diabetes
Even before the clinical diagnosis of T2DM, hypertriglyceridemia, low HDL-C, and elevated small dense LDL can be found in insulin-resistant, prediabetic people with acceptable glucose levels, so hyperglycemia cannot entirely account for the lipid alteration. Although the main cause of diabetic dyslipidemia is IR. 51 A key factor in determining the distinctive lipid profile of diabetic dyslipidemia is hypertriglyceridemia, which is regarded as the predominant lipid abnormality in insulin resistant individual. Increased synthesis and poor clearance of triglyceride-rich lipoproteins in both fasting and non-fasting stages lead to elevated triglyceride levels. IR involves an increased synthesis of very low-density lipoprotein cholesterol (VLDL-C), the primary carrier of fasting triglycerides. 52 VLDL-C can be divided into large, triglyceride-rich VLDL1 and small, dense VLDL2. VLDL 1 is reported to have greater triglyceride amount and has been found to be a significant predictor of plasma triglyceride levels as well as associated with insulin sensitivity. IR which is a key characteristic of T2DM, leads to dyslipidemia by several mechanisms. Insulin’s capacity to inhibit lipolysis is impaired by IR, increasing the release of FFA into the bloodstream, which in turn cause excessive production of VLDL-C by liver and carry triglycerides to the tissues in the periphery. As a result, this raises the amount of triglycerides in the blood.50,52,53 Hypertriglyceridemia is a result of both an increase in VLDL-C synthesis as well as their reduced clearance rate that is linked to postprandial triglyceride-rich chylomicrons, decreased hepatic uptake of VLDL-C, and impaired lipoprotein lipase activity. 54
Small, dense LDL becomes increasingly prevalent in T2DM patients and is more atherogenic than bigger LDL particles with a higher propensity to pierce the artery wall and is more vulnerable to oxidation, leading to the development of atherosclerotic plaques. Furthermore, the elevated cholesterol ester transfer protein (CETP) activity in hypertriglyceridemic patients facilitates the exchange of triglycerides from VLDL and LDL particles for cholesterol esters from HDL particles causing them to become enriched with triglycerides and depleted of cholesterol. As a result, these HDL particles become less effective at removing cholesterol from the bloodstream and also get more susceptible to degradation and clearance from the bloodstream. Consequently, the overall functionality of HDL particles is compromised, leading to lower levels of functional HDL cholesterol in T2DM patients. 55 Chronic low-grade inflammation also leads to aberrant lipid levels is a hallmark of T2DM. People with T2DM have higher levels of inflammatory mediators like CRP, IL-6, and TNF-α which worsens IR and encourages the development of hepatic VLDL particles resulting in dyslipidemia.56,57
Risk of CVD in diabetic dyslipidemia
Dyslipidemia is reported to elevate the risk of CVD in T2DM patients. Not only Hypertriglyceridemia, low HDL-C and elevated small dense LDL-C concentrations associate themselves in enhancing CVD risk. In comparison with non-diabetic people, T2DM patients might not show an elevated LDL-C concentration but have significantly greater number of small dense LDL particles, signifying the presence of greater atherogenic risk factor. 58 Even when LDL-C levels are below 130 mg/dl, the limit set by the National Cholesterol Education Program, LDL-C is still considered a potent independent predictor of CVD in T2DM. According to the Strong Heart Study, every 10 mg/dl rise in LDL-C was associated with a 12% increase in the risk of CVD in diabetes participants.59,60
As discussed earlier, atherosclerosis is most primary factor for the rise of any CVD that includes CAD, PAD and stroke. Dyslipidemia in T2DM promotes the development and progression of atherosclerosis by forming atherosclerotic plaques when levels of LDL-C, especially small dense LDL particles are elevated. 61 These small LDL-C particles can easily pass through the artery wall and are particularly susceptible to oxidation, encouraging the build up of cholesterol, inflammatory cells, and smooth muscle cells once penetrate the artery wall resulting in the development of fatty streaks followed by fibrous plaques. These plaques may rupture, developing blood clots that can result in MI or stroke. 62 Epidemiological and clinical research reports an inverse relationship between HDL-C concentration and CVD and considers HDL-C as the “good” lipoprotein due to its antiatherogenic function of reverse cholesterol transport as well as its cardioprotective functions that includes anti-inflammatory, anti-oxidative and endothelium-dependent vasodilatory effects and dyslipidemia is characterized with decreased HDL-C level.63,64 Endothelial function is also hampered by elevated LDL-C and other lipid abnormalities that encourage oxidative stress and inflammation in the arterial wall. The production of NO, a vasodialator is reduced in endothelial dysfunction, whereas the production of endothelin-1, a vasoconstrictor is enhanced thus unbalancing vascular tonicity.65,66 Additionally, dyslipidemia also affects the body’s fibrinolytic and anticoagulant mechanisms, which increase the probability of thrombus formation and makes blood clot dissolution less effective. 67 Figure 1 summarize the pathway depicting association between CVD and diabetic dyslipidemia. Alterations in lifestyle are essential for minimizing CVD risk and rebalancing lipid profiles that includes adhering to a healthy diet with diminished cholesterol, processed carbohydrates and saturated trans fats maintaining the body weight. 68 Improvement in insulin sensitivity, glycemic management, and lipid profile are linked to moderate weight loss (5% of body weight) and reportedly increase HDL-C and lowers triglyceride levels. Fruits, vegetables, whole grains, lean meats, and healthy fats are encouraged to be consumed along with regular exercise, such as strength training and aerobic workouts that improves insulin sensitivity, lowers triglyceride levels and elevates HDL-C level. 69
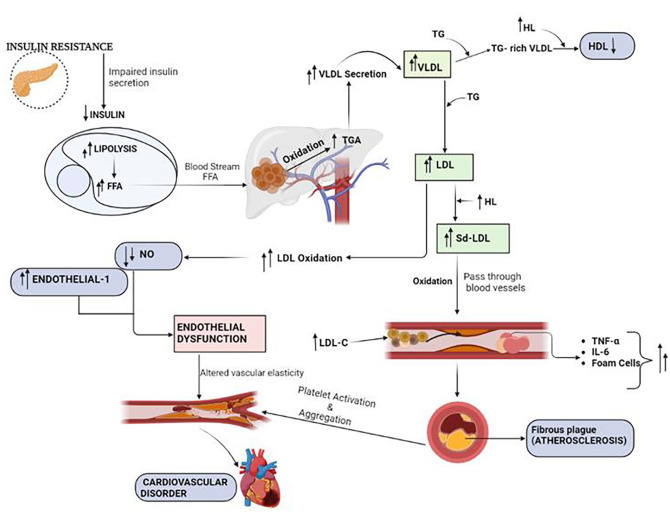
Figure 1.
Summarized pathway representing the manifestation of cardiovascular disorder due to dyslipidemia in patients with T2DM. Abbreviations: FFA, free fatty acid; HDL, high density lipoprotein; HL, hepatic lipoprotein; IL, interleukin; LDL, low density lipoprotein; LDL-C, low density lipoprotein cholesterol; NO, nitric oxide; Sd-LDL, small dense low density lipoprotein; TG/TGA, triglyceride; TNF-α, tumor necrosis factor-α; VLDL, very-low density lipoprotein.
Hypertension and Diabetes
A common medical condition called hypertension, also referred to as high blood pressure, is characterized by persistently raised blood pressure readings and is referred to as the “silent killer” is that it frequently exhibits no major clinical symptom but, if untreated, can result in fatal issues. Smoking, a sedentary lifestyle, and a high-sodium diet are all preliminary lifestyle factors that can cause hypertension. 70 T2DM and hypertension frequently coexist and have a strong association where both influences and exacerbates the other in the complex interaction. 71
Hypertension and diabetes
Hypertension impacted by a number of dysregulated physiological systems that elevates cardiac output and blood volume resulting in elevated blood pressure. T2DM is linked to a number of metabolic abnormalities, including IR, hyperglycemia, and dyslipidemia, which cause hypertension and in turn manifests CVD. 72 Diabetes-related metabolic complications, vascular disorder, and hormonal disorders interact intricately and elevates the blood pressure. 73 IR acts as a major factor in the emergence of hypertension by an array of interconnected mechanisms. Increased activity in the sympathetic nervous system is related to IR which arises because of defective insulin signaling to the regions of the brain that control sympathetic outflow. Also RAAS that controls and maintain electrolyte homeostasis, fluid balance, and blood pressure is compromised in faulty insulin signaling. 74
In insulin resistant condition, renin release from kidney is stimulated increasing the conversion of angiotensinogen into angiotensin I. Angiotensin-converting enzyme (ACE), abundantly present in lungs and blood vessels further converts angiotensin I into angiotensin II. As a powerful vasoconstrictor, angiotensin II induces blood vessel constriction and develop peripheral resistance, thus bringing up resistance in blood flow and elevating blood pressure.75,76 Further the secretion of aldosterone from the adrenal glands is also stimulated by angiotensin II that induces retention of both sodium and water expanding blood volume as a result blood pressure is raised. Angiotensin II also stimulate the sympathetic nervous system increasing the sympathetic activity which leads to excessive release of norepinephrine, enhancing vasoconstriction, and again elevates blood pressure.77,78 Adipokines, the bioactive chemicals by adipose tissue is unbalanced in IR and with increased pro-inflammatory adipokine and decreased anti-inflammatory adipokine production along with other hormonal changes, development of hypertension is manifested. Adiponectin is an adipokine with anti-inflammatory and insulin-sensitizing properties. Insulin resistant individuals frequently reports decreased adiponectin level which is associated with increased inflammation, poor vasodilation, and endothelial dysfunction, triggering hypertension.79,80 Another adipokine that affects appetite control and energy balance is leptin that is frequently elevated in obese and insulin resistant individual because of increased adipose tissue mass. Leptin levels when excessively elevated stimulates sympathetic nervous system, constricting blood vessels and elevates blood pressure. 81 Typically IR is associated with chronic low-grade inflammation in the body and inflammatory substances elevate vasoconstriction and impair blood vessel function, contributing to hypertension. 12 In normal physiological condition insulin increases endothelial NO synthesis but in IR the production of NO is downregulated leading to vasoconstriction and elevated blood pressure that ultimately contributes to the development of endothelial dysfunction. 82 T2DM is associated with increased sodium retention by the kidneys into the bloodstream which occurs due to insulin’s diminished effect on kidney’s renal tubules. As reabsorption of water increases along with sodium chloride, the blood volume expands and blood pressure elevates. Further T2DM includes hormonal imbalances, such as increased levels of insulin, cortisol (a stress hormone), and aldosterone which is studied to exert direct effects on blood pressure regulation by causing electrolyte imbalance in body.83,84 The sodium-potassium pump, a crucial mechanism for preserving cellular electrolyte balance, is activated by insulin. This pump’s function is compromised by IR causing intracellular sodium buildup and extracellular potassium depletion that triggers vasoconstriction, higher blood pressure and increased arterial stiffness ultimately triggering CVD. 85
Cardiovascular consequence of hypertension in diabetes
T2DM is the most common cause of end-stage renal disease, blindness, and nontraumatic amputations and is linked to several cardiovascular complications. Glycemia is the constant variable that serves as a cardiovascular risk factor and hypertension alone is a significant risk factor for cardiovascular morbidity and mortality and their coexistence in the same patient is detrimental. 86 Figure 2 summarize the overall impact and physiology of hypertension on the development of cardiovascular complication in presence of IR.
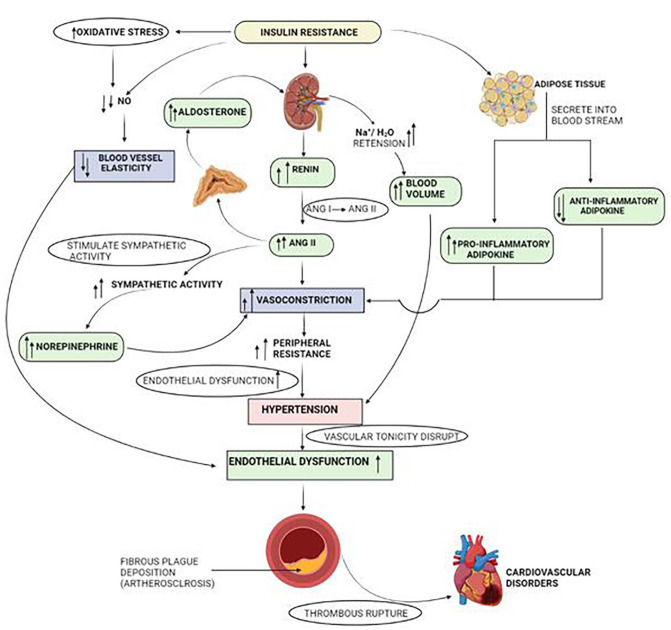
Figure 2.
Crossover pathways in hypertension and IR that lead to the development of CVD. Abbreviations: ANG-I, Angiotensin-I; ANG-II, Angiotensin-II; H2O, water; IR, insulin resistance; Na+, sodium ion; NO, nitric oxide; T2DM, Type 2 diabetes mellitus.
Impact on the vascular tree
T2DM and hypertension are both recognized contributors for atherogenesis and damage the vascular tree through an array of underlying factors. Endothelial dysfunction, a crucial early stage in the emergence of vascular problems in T2DM is exacerbated by hypertension. The endothelial cells that lines the blood arteries are subjected to greater degree of mechanical stress due to elevated blood pressure and compromise its capacity to control vascular tone, inflammation, and clotting factors, interfering with its standard functionality. 87 Hyperglycemia, a hallmark of T2DM generates ROS in excess that also damages the vascular tonicity of endothelial lining. Also oxidative stress produce AGEs which contribute to endothelial dysfunction and vascular damage in diabetes.88,89 The reduced availability of NO in endothelium in diabetic hypertensive condition is mediated by several factors. As was already established, elevated oxidative stress can inactivate NO by directly scavenging it. Furthermore, eNOS, the enzyme that produces NO is also impaired by high glucose levels. Vasoconstriction and endothelial dysfunction are caused by a combination of decreased production and increased degradation of NO ultimately enhancing peripheral resistance of the blood vessel and act as a contributing factor for atherosclerosis. 90 In diabetic hypertension the endothelial barrier breaks down increasing the permeability. Because of the malfunctioned endothelial cells, plasma proteins and lipids, particularly LDL-C leaks into the blood vessel, accumulating plaque for arthrosclerosis development. 91 In hypertension the endothelial cells are stimulated to produce endothelin-1 (ET-1), a strong vasoconstrictor, which tightens blood vessels and increases vascular resistance and excessive rise in blood pressure leads to vascular complications. 92
LDL-C and other circulating lipids that are drawn to the damaged endothelium builds up and undergoes oxidation within the artery wall, causing an inflammatory reaction and draws immune cells to the area notably macrophages and which releases pro-inflammatory cytokines. The formation of atherosclerotic plaque in the artery is brought on by the lipid engulfment of the macrophages, forming foam cell.93,94 The earliest detectable stage of atherosclerosis is a fatty stripe formed by inflammatory cells and lipid-filled foam cells over which a fibrous covering eventually forms as the fatty streak develops into an atheromatous plaque, a more complicated lesion. The unstable plaque with a thick lipid core and a thin fibrous cap is more likely to break and on rupture the highly thrombogenic components of the plaque, such as tissue factor and collagen, are made available to the bloodstream and platelets cling to the area formation blood clot, thereby obstructing the blood vessel that leads to ischemic events.95,96 Furthermore, heart muscle cells enlarges due to the increased stain on the artery walls and deposition of extracellular protein on leading to remodeling and hypertrophy of the bigger arteries in diabetic hypertension. The arterial stiffness, decreased compliance, and compromised vasodilation that follow these anatomical alterations elevates blood pressure and raise stress on heart. 97
Impact on the heart
As the atherosclerotic plaque developed within the artery wall ruptures that initiates a process of thrombus formation that block the coronary artery, decreasing blood flow to the heart muscle. Ischemia is the result of the narrowing or blockade of the heart arteries and if the blood flow is not quickly restored, it can result in symptoms like angina or more serious problems like MI. 98 The heart must work harder to pump blood against more resistance which result in chronic hypertension and develop hypertrophy of its muscular walls. The heart’s ability to pump blood decline with time resulting in heart failure or arrhythmias.99,100
Anomalies in the microcirculation are responsible for the severe degenerative alterations in the diabetic hypertensive heart. 101 Nonhemodynamic factors such as sodium intake, activity of growth-promoting hormones, activity of the sympathetic nervous system, RAAS, whole-blood viscosity glucose levels, and genetics contribute to the development of left ventricular hypertrophy that explains hypertensive cardiomyopathy in diabetic hypertension.102,103 The widespread presence of extensive interstitial connective tissue throughout the myocardium appears to be one of the most noticeable microscopic learning of the hypertensive diabetic heart. Clinical research using echocardiography also revealed that patients with diabetes and hypertension had an enlarged left ventricular mass.104,105 While nondiabetic hypertensive patients with comparable levels of hypertension only had a lower prevalence of left ventricular hypertrophy than hypertensive T2DM patients that partially clarify why T2DM patients have higher rates of morbidity and mortality. 106
Obesity and Diabetes
Obesity is a “chronic and multifactorial medical condition characterized by excessive accumulation of body fat, and is typically diagnosed based on an individual’s body mass index (BMI).” The World Health Organization (WHO) defines obesity as “abnormal or excessive fat accumulation that may impair health.” 107
Diabetes and obesity
As a significant contributor to IR, obesity raises plasma FFA levels over time, both in the basal state and after a glucose load. Central-abdominal fat has a higher level of metabolic and lipolytic activity, which results in a greater release of FFAs into the bloodstream. Whole-body IR was seen in clinical tests in healthy participants with acutely elevated plasma FFA levels. 108 Increased plasma FFAs impede glucose metabolism at the level of substrate competition, intermediates accumulation, enzyme regulation, intracellular signaling, and/or gene transcription by mass action to increase their cellular absorption and stimulate mitochondrial –oxidation. 109 As observed by the rise in lipid oxidation, the predominance of lipid utilization at the expense of glucose causes skeletal muscle to absorb less glucose and produce less glycogen at a slower rate. This chronic hyperglycemia (glucotoxicity) condition reduces insulin sensitivity substantially. 110 AGEs are produced as a result of the pathological glycation of circulating proteins and compensatory hyperinsulinemia carried on by IR and glucose intolerance resulting in pancreatic beta cell secretory failure are the consequences of this process in the final stages. 111 In addition to the liver, skeletal muscle, and pancreatic cells, which under normal circumstances do not store lipids, fat storage occurs inside and around these organs when the diet-derived fat intake is increased that ultimately leads to an excessive generation in the mitochondria of hazardous reactive lipid species that harm specific organs through oxidative stress and cellular malfunction, eventually developing into IR, poor glucose tolerance and T2DM. 112 An increasing amount of research points to the pathophysiology of diabetes in obese patients being affected by chronic low-grade inflammation in adipose tissue. 113 Through Lipopolysaccharide (LPS)-related endotoxemia that affects gut microbiota, obesity causes inflammation. An upsurge in T-lymphocytes and M1 macrophages along with enhanced production of proinflammatory cytokines induce inflamation and contribute to the development of IR. Additionally, inflammatory signals linked to obesity impair endoplasmic reticulum performance and significantly activate JNK, specifically the JNK1 isoform in insulin-sensitive organs like the liver and fat which functions by serine phosphorylation of the insulin receptor substrate-1 and subsequent suppression of insulin action.114,115
Cardiovascular consequences of obesity in T2DM
Hyperglycemia is brought on by poor glucose cellular uptake due to IR, where the formed AGEs rupture blood vessels and impair their function. Furthermore, increased plasma levels of FFAs in obese people aggravate IR and compromise glucose metabolism. 116 Low-grade inflammatory state developed in obesity is exacerbated in T2DM. Secretion of adipokines including leptin and resistin as well as pro-inflammatory cytokines encourage inflammation, promoting endothelial dysfunction. Also in obese T2DM condition elevated ROS weakens body’s antioxidant defense that induce oxidative damage to tissues, especially the vascular endothelium also contributing to endothelium dysfunction. 117 Endothelial dysfunction caused due to aforementioned condition induce hypertension and vascular injury as it decrease NO production, hence vascular tonicity disrupts. 118 Dyslipidemia in T2DM obese individual accelerates the development of atherosclerotic plaques within the arteries. These plaques may rupture or calcify over time, developing blood clots that partially or totally obstruct blood flow through the coronary arteries resulting in angina or MI. 119
Other than inflammatory cytokines, release of adipokine by adipose tissue in obese T2DM patient encourage sodium retention, vasoconstriction and enhance sympathetic nervous system activity that contribute to hypertension and systemic vascular resistance. 120 Chronic inflammation, oxidative stress, and IR in obese T2DM patient damage the heart muscle hampering cardiovascular cellular ability to utilize glucose, resulting in fatigue. Pro-inflammatory cytokines and adipokines also promote structural and functional alterations in the heart by increasing ventricular stiffness and impaired diastolic function. Heart failure develops as a result of reduced cardiac contractility caused by these variables taken together. Obesity and diabetes accelerates the developmental procedure of atherosclerosis, which can alter the cerebral arteries and limit blood flow along with the development of prothrombic condition. Uncontrolled diabetes also causes small vessel disease, such as cerebral small vessel disease and microangiopathy, which raises the risk of an ischemic stroke.121,122
Obese T2DM patients are more susceptible to CVD in which the heart undergoes structural and electrical remodeling. The normal electrical conduction is disrupted by the left ventricular hypertrophy, fibrosis, and dilatation that are linked to obesity. 123 Ion channel function and cardiac electrical activity are also affected by IR and dyslipidemia leading to cardiac arrhythmia. Pro-inflammatory cytokines also promote fibrosis in the heart, creating arrhythmogenic substrate. Identifying important pathways and prospective targets for treatments is made easier by having a thorough understanding of the pathophysiology of each cardiovascular disease in diabetes obesity.124,125 To slow the onset and progression of these cardiovascular problems, effective care should focus on managing diabetes, controlling obesity, managing hypertension, and addressing associated risk factors Figure 3 summarize the pathophysiology underlying the development of CVD in obese diabetic individuals.
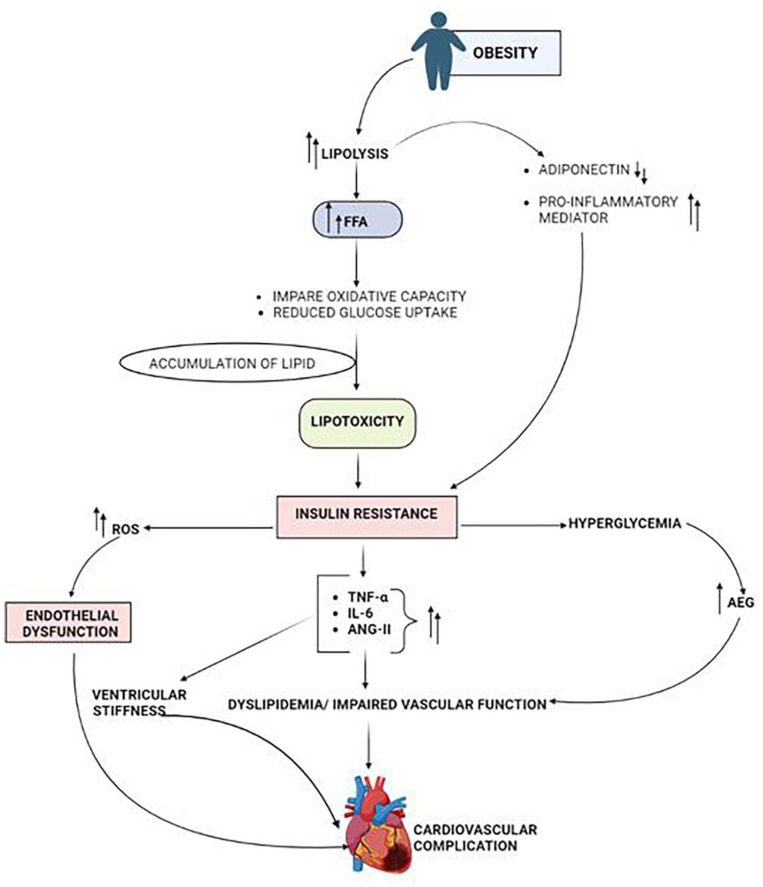
Figure 3.
Pathophysiology involved in the rise of CVD in obese diabetic individual. Abbreviations: AGE, advanced glycation end product; ANG-II, Angiotensin-II; FFA-Free fatty acid; IL6, interleukin6; ROS, Reactive Oxygen Species; TNF-α, tumor necrosis factor-α.
Diabetic Kidney Disease and Cardiovascular Health
The cardiovascular health is significantly impacted by diabetic kidney disease (DKD), also known as diabetic nephropathy, which is a common consequence of T2DM. A bidirectional association exists between DKD and cardiovascular disease, with each illness influencing the onset and advancement of the other.
Diabetic nephropathy
The prime pathological alteration in DKD occurs in kidneys’ glomeruli, renal tubules, and interstitium. In DKD, the filtering units of the kidneys, glomeruli experience pathogenic alterations where the glomerular filtration rate (GFR) and renal blood flow initially rise. This hyperfiltration appears to be a compensatory reaction to the raised glucose levels and increased metabolic demands associated with T2DM. 126 The glomeruli are under greater stress due to the elevated GFR and the glomerular filtration barrier becomes more permeable over time due hyperglycemia and GFR stress. Larger molecules, especially albumin leaks into urine due to this enhanced permeability causing albuminuria that is regarded as a glomerular filtration barrier degradation indicator and an early indicator of DKD.127,128 The glomerular basement membrane thickens as a result of buildup of Collagen and other elements of the extracellular matrix resulting in reduction of glomeruli’s capacity for filtration. 129
The accumulation of proteins, including type IV collagen develops Kimmelstiel-Wilson nodules and are the defining hallmark of advanced stage DKD. 130 The renal tubules enlarge due to fluid buildup and atrophy characterized by the loss of tubular cells and renal function is further compromised. The renal vasculature is potentially impacted by diabetes where arterioles supplying the kidneys thickens and narrows causing arteriolar hyalinosis contributing to renal ischemia and further kidney tissue injury. 131 It’s significant to remember that DKD pathophysiology is not exclusive to the kidneys. Organs and systems throughout the body are affected by diabetes and DKD, including Cardiovascular System. The development of hypertension, atherosclerosis and the increased risk of heart disease and stroke are all influenced by the pathophysiological alteration in the kidneys. 132
Linking cardiovascular disease and diabetic nephropathy
Endothelial dysfunction is linked to both DKD and CVD and due to prolonged hyperglycemia and metabolic imbalances. AGEs are produced in excess in hyperglycemia and activation of protein kinase C pathway worsens NO bioavailability, exacerbate oxidative stress and inflammation and manifest vasoconstriction. Ineffective vasodilation, increased arterial permeability and prothrombotic condition are developed endothelial dysfunction, which leads to atherosclerosis. 133 Due to the endothelial dysfunction in DKD, inflammatory cells including monocytes and lymphocytes are drawn to and adhere to the vascular endothelium. Secretion of adhesion molecules and chemokines by activated endothelium helps inflammatory cells move into the vessel wall and exude cytokines and growth factors encouraging development of extracellular matrix components and vascular smooth muscle cell proliferation. Atherosclerotic plaques develop as a result, narrowing the artery lumen, decreasing blood flow and consecutively raising the risk of CVD. 134
In DKD renin is released in excess from the juxtaglomerular cells as a result of poor sodium excretion and volume enlargement caused by renal injury. Angiotensin II is produced when RAAS is activated by renin that induces vasoconstriction, aldosterone release and sympathetic nervous system stimulation. These processes increase blood pressure, sodium and water retention and systemic vascular resistance. A vicious loop between renal impairment and cardiovascular issues is created by hypertension, which worsens already existing kidney disease. In addition to sodium and water retention, plasma volume expansion and raised blood pressure, Angiotensin II also increases the release of aldosterone that cause vascular remodeling and fibrosis by encouraging the growth of vascular smooth muscle cells. The risk of hypertension, atherosclerosis increases as a result of these modifications, which can result in heart failure and other cardiovascular problems.135,136 The production of numerous cytokines and growth factors in diabetic nephropathy together with systemic variables like hypertension, oxidative stress, inflammation and metabolic irregularities bring about structural alteration of the heart. These modifications include diastolic dysfunction, myocardial fibrosis, and left ventricular hypertrophy. 137 Myocardial fibrosis alters the natural structure of the myocardium, which impairs cardiac function and increases the risk of arrhythmias and heart failure. 138 A vicious cycle is created by the interaction of both DKD and T2DM with renal damage being exacerbated by cardiovascular disease while DKD promotes the growth and advancement of the ailment. To lessen the effects of DKD on cardiovascular health, effective management techniques should prioritize optimizing glycemic control, blood pressure management, cholesterol management, and renin-angiotensin system blocking. Figure 4 show the pathophysiological pathway of the development of CVD in diabetic nephropathy.
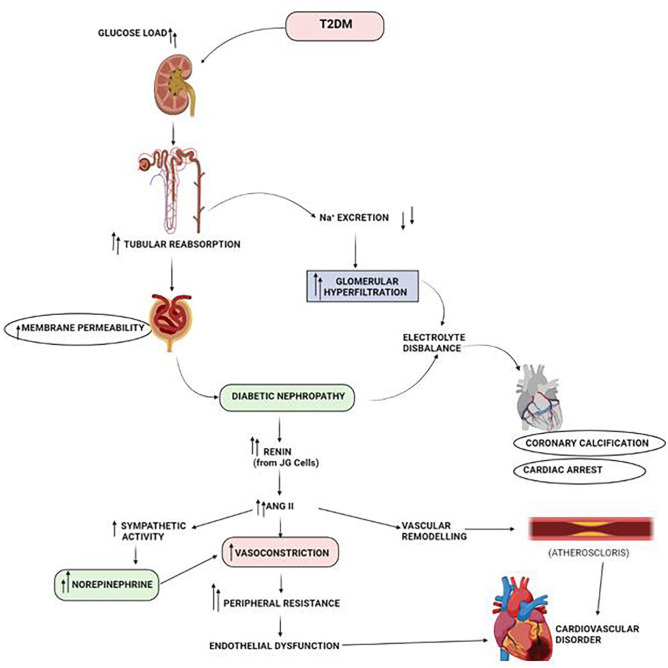
Figure 4.
Physiological pathway that lead to development of CVD in diabetic nephropathy patients. Abbreviations: ANG II- Angiotensin II; JG cells, Juxtaglomerular cells; Na+, sodium ion; T2DM, Type 2 diabetes mellitus.
Strategies for Managing Cardiometabolic Risks in Diabetes
Lifestyle modification
By practicing weight management, lowering inflammation, regulating blood sugar levels, controlling blood pressure and cholesterol levels, lifestyle changes have an impact on cardiometabolic diseases inT2DM, consistent implementation of which can greatly lower the probability of cardiovascular problems and enhance general wellbeing in T2DM patients. T2DM patients must consume complex carbohydrates, high-fiber foods, and spreading their carbohydrate intake throughout the day to avoid blood sugar spikes and maintain stable glucose levels. Physical activity improves insulin sensitivity, glucose uptake into cells, raise metabolism and maintain lipid profile that assist lowering hyperglycemia and obesity. The risk of cardiometabolic disorders is decreased when a healthy weight is maintained because it eases the load on the heart and blood vessels.139,140 People can minimize the risk of CVD and enhance blood vessel function by giving up smoking. 141 The release of stress hormones such cortisol, catecholamines and glucagon by the body during stress cause spike in blood sugar levels by boosting gluconeogenesis causing compromised glycemic control and IR in people with poor glucose metabolism. 142 The release of pro-inflammatory cytokines and the activation of immune cells are 2 features of low grade inflammatory reactions that chronic stress sets off in the body which is linked to the onset and development of CVD. 143 The duration and quality of sleep also affect the ratio of hormones that control appetite, such as leptin and ghrelin. Lack of sleep can interfere with these hormonal signals, causing cravings for high-calorie meals and increased hunger leading to obesity and lipotoxicity which are risk factors for T2DM. 144 In order to enhance glycemic control, lower IR, and lessen cardiovascular risks, comprehensive care for T2DM patients must include implementing effective stress management techniques and promoting healthy sleep habits.
Pharmacological interventions
In order to manage cardiovascular risk factors, regulate blood glucose levels, and lower the frequency of cardiovascular events, extensive pharmaceutical therapies are employed. The following section highlights the most widely used pharmaceutical treatments for T2DM’s cardiometabolic consequences.
Among oral antidiabetic medications the drug of choice for treating T2DM is metformin which promotes glucose absorption by peripheral tissues, raises insulin sensitivity, and decreases hepatic glucose synthesis. 145 Sulfonylureas like glibenclamide, glimepiride and glipizide encourage pancreatic β cells to secrete more insulin. 146 DPP-4 inhibitors that include sitagliptin, saxagliptin, and linagliptin improve glycemic management by increasing insulin production and decreasing glucagon release. 147 Sodium-Glucose Co-Transporter 2 (SGLT2) inhibitors including empagliflozin, dapagliflozin, and canagliflozin decrease renal glucose reabsorption and increases urine glucose excretion and improve glycemic management as well as lowers cardiovascular mortality. 148 Thiazolidinedione, in particular Pioglitazone are recommended as oral antidiabetic drug for increasing insulin sensitivity in peripheral tissues. 149 Other than oral medication several injectables are also prescribed for glycemic control. Exenatide, liraglutide, and dulaglutide imitate the effects of glucagon-like peptide-1 (GLP-1), a hormone that increases the secretion of the insulin and glucagon while inhibiting the release of glucagon, delaying gastric emptying and lowers the risk of CVD, GLP-1 receptor agonists have positive effects on the cardiovascular system. 150 Patients with T2DM who cannot achieve appropriate glycemic control with oral medicines require insulin therapy. 151 As part of the management of cardiometabolic problems in T2DM, antihypertensive medicines are frequently prescribed to control hypertension, a comorbidity that is frequently present in T2DM patients and lessen the load on the heart and blood vessels reducing the chances of CVD. To better manage blood pressure, many antihypertensive drugs of various kinds are frequently used in combination. When compared to a single medicine, combination therapy can reduce blood pressure more effectively due to its additive or synergistic effects. 152 Antihypertensive drug classes that are frequently prescribed include beta-blockers which majorly includes metoprolol and carvedilol that reduce heart rate and myocardial contractility, calcium channel blockers like amlodipine and verapamil that relax vascular smooth muscle cells, Angiotensin-converting enzyme inhibitors (ACE) and diuretics. 153 The most commonly preferred lipid lowering drug is statin, that primarily function by blocking synthesis of cholesterol, lowers LDL-C level and slightly raise HDL-C levels lowering the risk of atherosclerosis. Statins with its anti-inflammatory property help to stabilize plaque and prevents rupture, avoiding heart attacks and strokes. 154 Monoclonal antibodies evolocumab and alirocumab target proprotein convertase subtilisin/kexin type 9 (PCSK9) which breaks down LDL receptors and increase the number of LDL receptors thus increases LDL clearance and lowers the risk of CVD. 155 T2DM patients are more likely to develop thrombosis. Antiplatelet drugs like aspirin and cloperedogel supports healthy clotting process by preventing the development of thrombi inside blood vessels. Antiplatelet drugs are prescribed to T2DM patients having coronary artery stenting or other cardiovascular procedures in order to prevent stent thrombosis. 156 By lowering platelet activation and inflammation, antiplatelet medications not only reduce risk of CVD but also decrease progression of DKD.
Interplay of Cardiometabolic Disorders Manifesting Cardiovascular Complication in T2DM
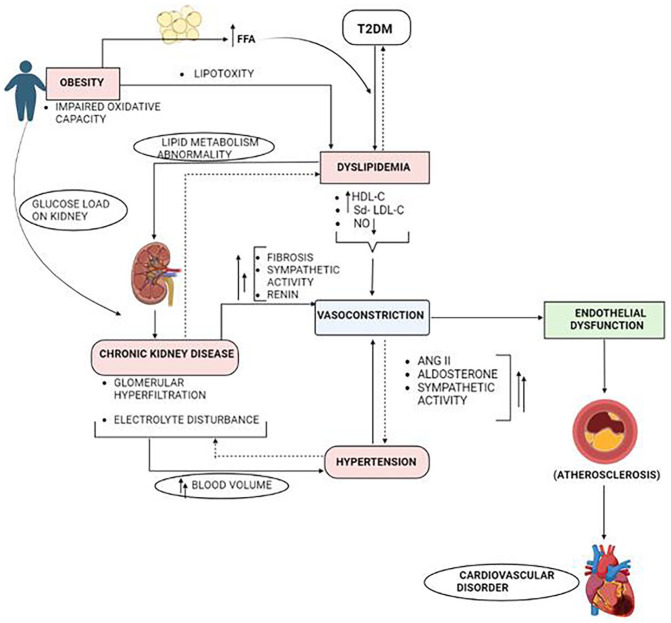
T2DM is characterized by IR and reduced insulin production and cardiometabolic diseases are frequently linked to it that increase the chance of developing CVD and is illustrated in Figure 5. Hyperglycemia is a result of poor glucose uptake caused by IR, which in turn initiates several pathophysiological processes. 157 By accelerating the release of free fatty acids from adipose tissue and decreasing the clearance of triglyceride-rich lipoproteins, it primarily encourages dyslipidemia that buildup of cholesterol-rich plaques in artery walls resulting in atherosclerosis.158,159 Furthermore, endothelial dysfunction, a crucial factor in CVD is triggered on by persistent hyperglycemia and dyslipidemia. 160 AGEs produced in hypertension encourage oxidative stress and inflammation and have the ability to bind to receptors on endothelial cells, initiating several signaling pathways that worsen endothelial dysfunction. 161 LDL-C particles in dyslipidemia enter the artery and cause inflammatory response and the oxidation of which leads to foam cell formation that progress atherosclerosis. 162 NO production is decreased in endothelial dysfunction, while endothelin-1, proinflammatory cytokines, and adhesion molecules are enhanced causing vasoconstriction, inflammation, and platelet aggregation exacerbating the onset and progression of cardiovascular problems.163,164 Furthermore, by encouraging the generation of ROS and decreasing antioxidant defenses, hyperglycemia and dyslipidemia heighten oxidative stress.
Figure 5.
Interconnection between different cardiometabolic complications that results in development of CVD in T2DM. Abbreviations: FFA, free fatty acid; HDL-C, high density lipoprotein cholesterol; NO, nitric oxide; Sd-LDL, small dense low density lipoprotein; T2DM, Type 2 diabetes mellitus.
TNF-, IL-6, and CRP are the inflammatory mediators that are produced in adipose tissue of obese T2DM patients that support IR, endothelial dysfunction and atherosclerosis by causing a persistent low-grade inflammatory state and promote plaque development. 165 IR causes the activation of sympathetic nervous system and cause electrolyte imbalance that raises blood pressure. 166 IR, dyslipidemia, atherosclerosis, endothelial dysfunction, oxidative stress, chronic inflammation, and hypertension are all part of the interrelated pathophysiology underpinning cardiometabolic illnesses that show cardiovascular consequences in T2DM. Cardiovascular problems in people with T2DM emerge and advance as a result of the interactions and amplifying effects of these processes.
Therapeutic Targets for Reducing the Mortality of T2DM Patients due to CVD
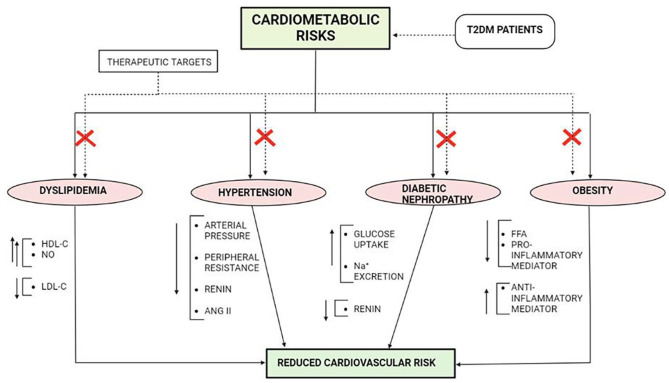
Over the past decade there has been a dramatic rise in the mortality of T2DM patients, mostly due to cardiovascular complications. According to International diabetes federation in 2019, approximately 4.2 million deaths are reported in T2DM due to CVD. 167 Epidemiological data supports that T2DM patients are at higher risk for incident of heart failure, MI and other cardiovascular complications. As discussed in earlier sections, IR is directly involved for the development of cardiovascular complications and several therapeutic interventions along with close glucose monitoring arresting the development of CVD. 167 Current research is focusing not only on glycemic control but arrest the cardiometabolic disorders of an individual can be an ideal way to reduce the mortality of T2DM patients by CVD. Figure 6 represents the major therapeutic targets that can effectively control the risk of development of CVD in T2DM patients.
Figure 6.
Major therapeutic targets for the management of cardiovascular disorder in T2DM patient. Abbreviations: ANG II, Angiotensin II; FFA, free fatty acid; HDL, high density lipoprotein; LDL, low density lipoprotein; NO, nitric oxide; T2DM, Type 2 diabetes mellitus.
Studies suggest that there exist a missing link that explains the excessive burden of CVD in T2DM patients despite being under optimal glycemic control. The cardiometabolic factors play underlying role in the manifestation of abrupt development of endothelial dysfunction, severe vasoconstriction and ventricular dysfunction that ultimately impairs cardiovascular functionality. 168 Several study reports that individual suffering from hypertension tends to show 50% to 60% mortality due to sudden heart attack. When this elevated condition of blood pressure associates with IR, the development of cardiovascular disease grows rapidly. Identifying hypertension as a therapeutic target to reduce risk of CVD is an important measure to deplete mortality rate. On controlling hypertensive state in individual, the arterial pressure and peripheral resistance reduces, thus reducing vasoconstriction, thereby reducing damage of vascular physiology. Also reduction of blood volume decrease cardiac output and load on heart decreases, thereby reducing risk of CVD development.169,170 On the other hand targeting to reduce dyslipidemia directly reduce the risk of cardiovascular complication. Study suggests that dyslipidemia and risk of CVD runs parallel, increasing the incidence of atherosclerosis by 2 to 3 folds. 171 With almost 70% T2DM patient having LDL-C level of >100mg/dl, dyslipidemia can be considered a prominent therapeutic target to reduce the T2DM mortality by CVD. The reduction in LDL-C release and upregulation of HDL-C balances TG level and reduce chance of plaque deposition in the artery walls. Further the increased NO level balances vasodialation thus keeping the vascular elasticity intact and downslide the chances of development of cardiovascular complications. 172 Another therapeutic target for achieving intact and healthy vascular function in T2DM patient with high BMI is management of body weight. Obese individual having high BMI have an hazardous effect on cardiovascular health increasing risk of coronary heart disease upto 28%, even if individual is reported to show healthy blood pressure, controlled glycemia and cholesterol level. 173 Obesity is associated with not only development of CVD but also it acts as an underlying mechanism for the development of IR and hyperglycemia, as well as lipotoxicity leading to atherosclerosis and impaired vascular functionality. Obese patients if primarily treated to check body mass and BMI other than glycemic control can be at lower risk for CVD development. 173 Another prime therapeutic target is the treatment of diabetic nephropathy that disturbs the electrolyte balance in the body. Healthy functioning of kidney reduces cardiac arrhythmia by reducing systemic inflammation, balancing vasodialation and thus reducing risk of CVD development. There exist several drug classes that effectively reduce cardiometabolic factors individually. But introduction of combination drug regimen for T2DM patients with metabolic complications can be considered an important therapeutic intervention to control the mortality of T2DM patients due to cardiovascular complications.
Conclusion
The exploration of cardiometabolic risk factors associated with T2DM provides crucial insights into the intricate mechanisms underlying this complex and prevalent condition. Through comprehensive research and analysis, it has become evident that a multifaceted interplay of genetic, environmental, and lifestyle factors contributes significantly to the development of T2DM, the identification of which pave way for advancements in prevention, early detection, and management strategies. Mechanistic insights into the relationship between cardiometabolic risk factors and T2DM have illuminated pathways involving hypertension, dyslipidemia, obesity and diabetic nephropathy that not only deepen our comprehension of the disease but also offer potential targets for therapeutic interventions. These cardiometabolic risk factors having interconnected pathway often pave way for extreme metabolic condition and induce multiple complication in individual. When conjoined with IR, the metabolic conditions manifest the progression of plaque building in arterial wall, enhance vasoconstriction, disrupt vascular tonicity and also multiply cardiac output thus exposing T2DM patients at high risk of cardiovascular mortality. Other than close glycemic control, therapeutic regulation of cardiometabolic condition in T2DM patients can be considered as novel therapeutic targets to lower chances of cardiovascular mortality and provide healthy life to the patients.
The review article on cardiometabolic risk factors associated with T2DM stands out for its comprehensive approach, encompassing a vast array of literature and present complex mechanistic pathways in an accessible manner. It adeptly identifies and discusses novel connections between cardiometabolic risk factors and T2DM, contributing significantly to the field’s advancement. However, the review does have limitations that involve its vast scope, potentially leading to the omission of certain relevant studies and findings, limiting its comprehensiveness. Moreover, there might be a bias in the selection of data sources, impacting the review’s objectivity. While the article explores clinical implications, the practical application of mechanistic insights in real-world patient care settings is not thoroughly discussed. Additionally, the review does not extensively delve into future research directions, and the heterogeneity of study populations across reviewed studies might not have been fully addressed. Despite these limitations, acknowledging the review’s strengths and weaknesses is crucial for a balanced evaluation, guiding both researchers and readers in their understanding of cardiometabolic risk factors in T2DM.
Acknowledgments
Authors would like to thank Overseas Health Care Pvt. Ltd, Phillaur for providing necessary facilities for completion of this work.
Declarations
Ethics Approval and Consent to Participate: Not applicable because present work is a review article which don’t involve any human or animal subjects.
Consent for Publication: All authors have approved the final version of this article and given consent for publication.
Author Contributions: Snigdha Chakraborty: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing. Anjali Verma: Conceptualization; Data curation; Formal analysis; Visualization; Writing – review & editing. Rajeev Garg: Conceptualization; Data curation; Formal analysis; Validation; Writing – review & editing. Jyoti Singh: Conceptualization; Formal analysis; Methodology; Validation; Writing – review & editing. Hitesh Verma: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: Data used for compilation of present work is available with corresponding author (Dr. Hitesh Verma) and can be obtained upon request.
References
- 1. Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600-609. [DOI] [PubMed] [Google Scholar]
- 2. Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff. 2007;26:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dal Canto E, Ceriello A, Rydén L, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25-32. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Yang H, Yang P. The correlation between type 2 diabetes mellitus and cardiovascular disease risk factors in the elderly. Appl Bionics Biomech. 2022;2022:4154426-4154427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Vanuzzo D, Pilotto L, Mirolo R, et al. [Cardiovascular risk and cardiometabolic risk: an epidemiological evaluation]. G Ital Cardiol. 2008;9:6S-17S. [PubMed] [Google Scholar]
- 8. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp. 2013;61:119-125. [DOI] [PubMed] [Google Scholar]
- 9. Janiszewski PM, Ross R. The utility of physical activity in the management of global cardiometabolic risk. Obesity. 2009;17 Suppl 3:S3-S14. [DOI] [PubMed] [Google Scholar]
- 10. Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491-510, vii. [DOI] [PubMed] [Google Scholar]
- 11. Asada Y, Yamashita A, Sato Y, Hatakeyama K. Pathophysiology of atherothrombosis: mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol Int. 2020;70:309-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancusi C, Izzo R, di Gioia G, et al. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev. 2020;27:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Chi X, Wang Y, et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuíñas A, García-Morales V, Viña D, Gil-Longo J, Campos-Toimil M. Activation of PKA and epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci. 2016;155:102-109. [DOI] [PubMed] [Google Scholar]
- 15. Pearson T, Wattis JA, King JR, MacDonald IA, Mazzatti DJ. The effects of insulin resistance on individual tissues: an application of a mathematical model of metabolism in humans. Bull Math Biol. 2016;78:1189-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steiner DF, Park S-Y, Støy J, Philipson LH, Bell GI. A brief perspective on insulin production. Diabetes Obes Metab. 2009;11 Suppl 4:189-196. [DOI] [PubMed] [Google Scholar]
- 17. Boland BB, Rhodes CJ, Grimsby JS. The dynamic plasticity of insulin production in β-cells. Mol Metab. 2017;6:958-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Czech MP. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab. 2020;34:27-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021;22:751-771. [DOI] [PubMed] [Google Scholar]
- 20. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang F, Chen J, Wang J, Zhu P, Lin W. Palmitic acid induces MicroRNA-221 expression to decrease glucose uptake in HepG2 cells via the PI3K/AKT/GLUT4 pathway. Biomed Res Int. 2019;2019:8171989-8171998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Mrabeh A. Pathogenesis and remission of type 2 diabetes: what has the twin cycle hypothesis taught us? Cardiovasc Endocrinol Metab. 2020;9:132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64-80. [DOI] [PubMed] [Google Scholar]
- 25. Hsueh WA, Anderson PW. Hypertension, the endothelial cell, and the vascular complications of diabetes mellitus. Hypertension. 1992;20:253-263. [DOI] [PubMed] [Google Scholar]
- 26. Aronson D, Edelman ER. Coronary artery disease and diabetes mellitus. Cardiol Clin. 2014;32:439-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haas AV, McDonnell ME. Pathogenesis of cardiovascular disease in Diabetes. Endocrinol Metab Clin North Am. 2018;47:51-63. [DOI] [PubMed] [Google Scholar]
- 28. Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9:830-834. [DOI] [PubMed] [Google Scholar]
- 29. Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779-788. [DOI] [PubMed] [Google Scholar]
- 30. Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med. 2003;115 Suppl 8A:24S-28S. [DOI] [PubMed] [Google Scholar]
- 31. Lainampetch J, Panprathip P, Phosat C, et al. Association of tumor necrosis factor alpha, interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: a retrospective cohort study of rural Thais. J Diabetes Res. 2019;2019:9051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen MT, Pham I, Valensi P, et al. Flow-mediated-paradoxical vasoconstriction is independently associated with asymptomatic myocardial ischemia and coronary artery disease in type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herlitz J, Malmberg K, Karlson BW, Rydén L, Hjalmarson A. Mortality and morbidity during a five-year follow-up of diabetics with myocardial infarction. Acta Med Scand. 1988;224:31-38. [DOI] [PubMed] [Google Scholar]
- 34. Meerarani P, Badimon JJ, Zias E, Fuster V, Moreno PR. Metabolic syndrome and diabetic atherothrombosis: Implications in vascular complications. Curr Mol Med. 2006;6:501-514. [DOI] [PubMed] [Google Scholar]
- 35. Garg P, Kumar P, Shakya K, et al. Detection of brain stroke using electroencephalography (EEG). In: 13th International Conference on Sensing Technology (ICST). IEEE;2019:1–6. [Google Scholar]
- 36. Khoury JC, Kleindorfer D, Alwell K, et al. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke. 2013;44:1500-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8:325-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77-82. [Google Scholar]
- 39. da Silva RMFL. Influence of inflammation and atherosclerosis in atrial fibrillation. Curr Atheroscler Rep. 2017;19:2. [DOI] [PubMed] [Google Scholar]
- 40. Asghar O, Alam U, Hayat SA, et al. Obesity, diabetes and atrial fibrillation; epidemiology, mechanisms and interventions. Curr Cardiol Rev. 2012;8:253-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tuttolomondo A. Relationship between diabetes and ischemic stroke: analysis of diabetes- related risk factors for stroke and of specific patterns of stroke associated with diabetes mellitus. Diabetes Metab. 2015:5. doi: 10.4172/2155-6156.100054425457474 [DOI] [Google Scholar]
- 42. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333-3341. [DOI] [PubMed] [Google Scholar]
- 43. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921-929. [DOI] [PubMed] [Google Scholar]
- 44. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340. [DOI] [PubMed] [Google Scholar]
- 45. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581. [DOI] [PubMed] [Google Scholar]
- 46. Price JF, Lee AJ, Fowkes FG. Hyperinsulinaemia: a risk factor for peripheral arterial disease in the non-diabetic general population. J Cardiovasc Risk. 1996;3:501-505. [PubMed] [Google Scholar]
- 47. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425-428. [DOI] [PubMed] [Google Scholar]
- 48. Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998-1005. [DOI] [PubMed] [Google Scholar]
- 49. Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arca M, Pigna G, Favoccia C. Mechanisms of diabetic dyslipidemia: relevance for atherogenesis. Curr Vasc Pharmacol. 2012;10:684-686. [DOI] [PubMed] [Google Scholar]
- 51. Lorenzo C, Hartnett S, Hanley AJ, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98:1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Adiels M, Olofsson S-O, Taskinen M-R, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225-1236. [DOI] [PubMed] [Google Scholar]
- 53. Adiels M, Borén J, Caslake MJ, et al. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:1697-1703. [DOI] [PubMed] [Google Scholar]
- 54. Saleh J, Sniderman AD, Cianflone K. Regulation of plasma fatty acid metabolism. Clin Chim Acta. 1999;286:163-180. [DOI] [PubMed] [Google Scholar]
- 55. Jin X, Yang S, Lu J, Wu M. Small, dense low-density lipoprotein-cholesterol and atherosclerosis: relationship and therapeutic strategies. Front Cardiovasc Med. 2021;8:804214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Son D-H, Ha H-S, Park H-M, et al. New markers in metabolic syndrome. Adv Clin Chem. 2022;110:37-71. [DOI] [PubMed] [Google Scholar]
- 57. Jenkins AJ. Links between glucose and lipoproteins. In: Jenkins AJ, Toth PP. eds. Lipoproteins in Diabetes Mellitus. Springer International Publishing;2015:33-54. [Google Scholar]
- 58. Sugden M, Holness M. Pathophysiology of diabetic dyslipidemia:implications for atherogenesis and treatment. Clin Lipidol. 2011;6:401-411. [Google Scholar]
- 59. Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiol Rev. 2006;14:125-135. [DOI] [PubMed] [Google Scholar]
- 60. Howard BV, Robbins DC, Sievers ML, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the strong heart study. Arterioscler Thromb Vasc Biol. 2000;20:830-835. [DOI] [PubMed] [Google Scholar]
- 61. Li Y, Liu Y, Liu S, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fogelstrand P, Borén J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr Metab Cardiovasc Dis. 2012;22:1-7. [DOI] [PubMed] [Google Scholar]
- 63. Bruckert E, Hansel B. HDL-c is a powerful lipid predictor of cardiovascular diseases: HDL-c and cardiovascular diseases. Int J Clin Pract. 2007;61:1905-1913. [DOI] [PubMed] [Google Scholar]
- 64. Banerjee Y, Patti AM, Giglio RV, et al. The role of atherogenic lipoproteins in diabetes: molecular aspects and clinical significance. J Diabetes Complications. 2023:108517. [DOI] [PubMed] [Google Scholar]
- 65. Ataie Z, Fatehi-Hassanabad Z, Nakhaee S, Foadoddini M, Farrokhfall K. Sex-specific endothelial dysfunction induced by high-cholesterol diet in rats: the role of protein tyrosine kinase and nitric oxide. Nutr Metab Cardiovasc Dis. 2022;32:745-754. [DOI] [PubMed] [Google Scholar]
- 66. Andreadi A, Bellia A, Di Daniele N, et al. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: a target for new therapies against cardiovascular diseases. Curr Opin Pharmacol. 2022;62:85-96. [DOI] [PubMed] [Google Scholar]
- 67. Lin Q, Yang G, Ruan J, et al. Study of the significance of thromboelastography changes in patients with dyslipidemia. Emerg Med Int. 2022;2022:1927881-1927886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68. Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: A meta-analysis. PLoS One. 2020:e0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ngayimbesha A, Bizimana Jean B, Gakima Marie S. Effect of eight weeks of exercise training on lipid profile and insulin sensitivity in obese person. Int J Sports Exerc Med. 20199:119 . doi: 10.23937/2469-5718/1510119 [DOI] [Google Scholar]
- 70. O’Shea PM, Griffin TP, Fitzgibbon M. Hypertension: the role of biochemistry in the diagnosis and management. Clin Chim Acta. 2017;465:131-143. [DOI] [PubMed] [Google Scholar]
- 71. Tian X, Zuo Y, Chen S, et al. Hypertension, arterial stiffness, and diabetes: a prospective cohort study. Hypertension. 2022;79:1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sinha S, Haque M. Insulin resistance is cheerfully hitched with hypertension. Life. 2022;12:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Law JP, Pickup L, Pavlovic D, Townend JN, Ferro CJ. Hypertension and cardiomyopathy associated with chronic kidney disease: epidemiology, pathogenesis and treatment considerations. J Hum Hypertens. 2023;37:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Seravalle G, Grassi G. Renin–angiotensin–aldosterone system and blood pressure regulation. In: Pappachan JM, Fernandez CJ eds. Endocrine Hypertension. Elsevier;2023:63-75. [Google Scholar]
- 75. Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16:120-126. [DOI] [PubMed] [Google Scholar]
- 76. Chen YL, Sonkusare SK. Mechanosensitive angiotensin II receptor signaling in pressure-induced vasoconstriction. J Am Heart Assoc. 2022:e024740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kant R, Gupta S, Kumra T, et al. Role of renin angiotensin-aldosterone system in kidney homeostasis. In: Bhullar SK, Tappia PS, Dhalla NS. eds. The Renin Angiotensin System in Cancer, Lung, Liver and Infectious Diseases. Springer International Publishing;2023:245-259. [Google Scholar]
- 78. Garcia Fragas M, Gastão Davanzo G. Cross-talk between TNF-α and angiotensin II in the neural control of hypertension. J Neurosci. 2021;41:7512-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murakami H, Ura N, Furuhashi M, et al. Role of adiponectin in insulin-resistant hypertension and atherosclerosis. Hypertens Res. 2003;26:705-710. [DOI] [PubMed] [Google Scholar]
- 81. Russo B, Menduni M, Borboni P, Picconi F, Frontoni S. Autonomic nervous system in obesity and insulin-resistance-the complex interplay between leptin and central nervous system. Int J Mol Sci. 2021;22:5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muniyappa R, Chen H, Montagnani M, Sherman A, Quon MJ. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am J Physiol Endocrinol Metab. 2020;319:E629-E646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oltmanns KM, Dodt B, Schultes B, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325-331. [DOI] [PubMed] [Google Scholar]
- 84. Tanaka M. Improving obesity and blood pressure. Hypertens Res. 2020;43:79-89. [DOI] [PubMed] [Google Scholar]
- 85. Tack CJ, Lutterman JA, Vervoort G, Thien T, Smits P. Activation of the sodium-potassium pump contributes to insulin-induced vasodilation in humans. Hypertension. 1996;28:426-432. [DOI] [PubMed] [Google Scholar]
- 86. Winer N, Sowers JR. Epidemiology of diabetes. J Clin Pharmacol. 2004;44:397-405. [DOI] [PubMed] [Google Scholar]
- 87. Gamrat A, Surdacki MA, Chyrchel B, Surdacki A. Endothelial dysfunction: a contributor to adverse cardiovascular remodeling and heart failure development in type 2 diabetes beyond accelerated atherogenesis. J Clin Med. 2020;9:2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jamwal S, Sharma S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res. 2018;67:391-405. [DOI] [PubMed] [Google Scholar]
- 89. Yamagishi SI, Maeda S, Matsui T, et al. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663-671. [DOI] [PubMed] [Google Scholar]
- 90. Nikolaeva M, Johnstone M. Nitric oxide, its role in diabetes mellitus and methods to improve endothelial function. In: Johnstone M, Veves A. eds. Diabetes and Cardiovascular Disease. Springer International Publishing; 2023:159-200. [Google Scholar]
- 91. Ioannidou A, Fisher RM, Hagberg CE. The multifaceted roles of the adipose tissue vasculature. Obes Rev. 2022:e13403. doi: 10.1111/obr.13403 [DOI] [PubMed] [Google Scholar]
- 92. Little PJ, Ivey ME, Osman N. Endothelin-1 actions on vascular smooth muscle cell functions as a target for the prevention of atherosclerosis. Curr Vasc Pharmacol. 2008;6:195-203. [DOI] [PubMed] [Google Scholar]
- 93. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5:927-946. [PMC free article] [PubMed] [Google Scholar]
- 94. Singh S, Changkija S, Mudgal R, Ravichandiran V. Bioactive components to inhibit foam cell formation in atherosclerosis. Mol Biol Rep. 2022;49:2487-2501. [DOI] [PubMed] [Google Scholar]
- 95. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. 2023:1098725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duran MC, Mas S, Martin-Ventura JL, et al. Proteomic analysis of human vessels: application to atherosclerotic plaques. Proteomics. 2003;3:973-978. [DOI] [PubMed] [Google Scholar]
- 97. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864-886. [DOI] [PubMed] [Google Scholar]
- 98. Bäck M, Yurdagul A, Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boland J, Muller DWM. eds. Interventional Cardiology and Cardiac Catheterisation: The Essential Guide. 2nd ed. CRC Press/Taylor and Francis Group; 2019. [Google Scholar]
- 100. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117:2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med. 2020;9:2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Burns J, Ball SG, Worthy G, et al. Hypertensive left ventricular hypertrophy: a mechanistic approach to optimizing regression assessed by cardiovascular magnetic resonance. J Hypertens. 2012;30:2039-2046. [DOI] [PubMed] [Google Scholar]
- 103. Abdel Ghafar MT. Association of aldosterone synthase CYP11B2 (-344C/T) gene polymorphism with essential hypertension and left ventricular hypertrophy in the Egyptian population. Clin Exp Hypertens. 2019;41:779-786. [DOI] [PubMed] [Google Scholar]
- 104. Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810-1834. [DOI] [PubMed] [Google Scholar]
- 105. Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nasir T, Abbas S, Khalil U, et al. A comparative change in left ventricular mass index on echocardiography in hypertensive diabetic and non-diabetic patients taking candesartan. Pak J Med Heal Sci. 2023;17:569-572. [Google Scholar]
- 107. Müller MJ, Geisler C. Defining obesity as a disease. Eur J Clin Nutr. 2017;71:1256-1258. [DOI] [PubMed] [Google Scholar]
- 108. Westphal SA. Obesity, abdominal obesity, and insulin resistance. Clin Cornerstone. 2008;9:23-29. [DOI] [PubMed] [Google Scholar]
- 109. Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299-314. [DOI] [PubMed] [Google Scholar]
- 110. Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930-E934. [DOI] [PubMed] [Google Scholar]
- 111. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Investig. 2006;116:1802-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201-229. [DOI] [PubMed] [Google Scholar]
- 113. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes. 2008;32:S52-S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pereira SS, Alvarez-Leite JI. Low-grade inflammation, obesity, and diabetes. Curr Obes Rep. 2014;3:422-431. [DOI] [PubMed] [Google Scholar]
- 116. Felber J-P, Golay A. Pathways from obesity to diabetes. Int J Obes. 2002;26:S39-S45. [DOI] [PubMed] [Google Scholar]
- 117. Borilova Linhartova P, Janos J, Poskerova H, et al. Adipokine gene variability and plasma levels in patients with chronic periodontitis -a case-control study. Braz Oral Res. 2019;33:e034. [DOI] [PubMed] [Google Scholar]
- 118. Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr Clin Res Rev. 2019;13:1165-1172. [DOI] [PubMed] [Google Scholar]
- 119. Zhang T, Chen J, Tang X, et al. Interaction between adipocytes and high-density lipoprotein:new insights into the mechanism of obesity-induced dyslipidemia and atherosclerosis. Lipids Health Dis. 2019;18:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35:4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ren J, Wu NN, Wang S, Sowers JR, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101:1745-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Maida CD, Daidone M, Pacinella G, et al. Diabetes and ischemic stroke: an old and new relationship an overview of the close interaction between these diseases. Int J Mol Sci. 2022;23:2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Middeldorp ME, Kamsani SH, Sanders P. Obesity and atrial fibrillation: prevalence, pathogenesis, and prognosis. Prog Cardiovasc Dis. 2023;78:34-42. [DOI] [PubMed] [Google Scholar]
- 124. Delisle BP, Aromolaran AS. New insights into cardiac ion channel regulation 2.0. Int J Mol Sci. 2023;24:4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Krishnan A, Sharma H, Yuan D, Trollope AF, Chilton L. The role of epicardial adipose tissue in the development of atrial fibrillation, coronary artery disease and chronic heart failure in the context of obesity and Type 2 diabetes mellitus: a narrative review. J Cardiovasc Dev Dis. 2022;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang Y, Xu G. Update on pathogenesis of glomerular hyperfiltration in early diabetic kidney disease. Front Endocrinol. 2022:872918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lewko B, Stepinski J. Hyperglycemia and mechanical stress: targeting the renal podocyte. J Cell Physiol. 2009;221:288-295. [DOI] [PubMed] [Google Scholar]
- 128. García-Carro C, Vergara A, Bermejo S, et al. How to assess diabetic kidney disease progression? From albuminuria to GFR. J Clin Med. 2021;10:2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gilbert RE, Cooper ME, Jerums G. Extracellular matrix, growth factors and their interactions in the pathogenesis of diabetic kidney disease. Nephrology. 1996;2:291-303. [Google Scholar]
- 130. Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Casp J Intern Med. 2015;6:120-127. [PMC free article] [PubMed] [Google Scholar]
- 132. Marshall SM. Recent advances in diabetic nephropathy. Postgrad Med J. 2004;80:624-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen J, Liu Q, He J, Li Y. Immune responses in diabetic nephropathy: pathogenic mechanisms and therapeutic target. Front Immunol. 2022:958790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144-152. [DOI] [PubMed] [Google Scholar]
- 136. Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16:431. [DOI] [PubMed] [Google Scholar]
- 137. Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693-700. [DOI] [PubMed] [Google Scholar]
- 139. Papakonstantinou E, Oikonomou C, Nychas G, Dimitriadis GD. Effects of diet, lifestyle, chrononutrition and alternative dietary interventions on postprandial glycemia and insulin resistance. Nutrients. 2022;14:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sato Y, Nagasaki M, Kubota M, Uno T, Nakai N. Clinical aspects of physical exercise for diabetes/metabolic syndrome. Diabetes Res Clin Pract. 2007;77 Suppl 1:S87-S91. [DOI] [PubMed] [Google Scholar]
- 141. Khoramdad M, Vahedian-Azimi A, Karimi L, et al. Association between passive smoking and cardiovascular disease: a systematic review and meta-analysis. IUBMB Life. 2020;72:677-686. [DOI] [PubMed] [Google Scholar]
- 142. Kuo T, McQueen A, Chen T-C, et al. Regulation of glucose homeostasis by glucocorticoids. In: Wang J-C, Harris C. eds. Glucocorticoid Signaling. Springer;2023:99-126. [Google Scholar]
- 143. Silveira Rossi JL, Barbalho SM, Reverete de, Araujo R, et al. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab Res Rev. 2022:e3502. doi: 10.1002/dmrr.3502 [DOI] [PubMed] [Google Scholar]
- 144. Sharma S, Kavuru M. Sleep and metabolism: an overview. Int J Endocrinol. 2010;2010:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Shurrab NT, Arafa E-SA. Metformin: a review of its therapeutic efficacy and adverse effects. Obes Med. 2020:100186. [Google Scholar]
- 146. Volke V, Katus U, Johannson A, et al. Systematic review and meta-analysis of head-to-head trials comparing sulfonylureas and low hypoglycaemic risk antidiabetic drugs. BMC Endocr Disord. 2022;22:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yaribeygi H, Atkin SL, Sahebkar A. Natural compounds with DPP-4 inhibitory effects: Implications for the treatment of diabetes. J Cell Biochem. 2019;120:10909-10913. [DOI] [PubMed] [Google Scholar]
- 148. Brown E, Wilding JPH, Alam U, et al. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53: 2072-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yoo J, Jeon J, Baik M, Kim J. Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study. Cardiovasc Diabetol. 2023;22:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Akbarian M. Insulin therapy; a valuable legacy and its future perspective. Int J Biol Macromol. 2021;181:1224-1230. [DOI] [PubMed] [Google Scholar]
- 152. Wright AK, Kontopantelis E, Emsley R, et al. Cardiovascular risk and risk factor management in type 2 diabetes mellitus: a population-based cohort study assessing sex disparities. Circulation. 2019;139:2742-2753. [DOI] [PubMed] [Google Scholar]
- 153. Marjina Singh A, Sharma A, Narang RK, Singh G. Management of hypertension with conventional and herbals drugs. J Drug Deliv Therapeut. 2020;10:280-287. [Google Scholar]
- 154. Egom EEA, Hafeez H. Biochemistry of statins. Adv Clin Chem. 2016;73:127-168. [DOI] [PubMed] [Google Scholar]
- 155. Chapman MJ, Stock JK, Ginsberg HN. PCSK9 inhibitors and cardiovascular disease: heralding a new therapeutic era. Curr Opin Lipidol. 2015;26:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Grove EL, Gregersen S. Antiplatelet therapy in patients with diabetes mellitus. Curr Vasc Pharmacol. 2012;10:494-505. [DOI] [PubMed] [Google Scholar]
- 157. Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clin Cornerstone. 2007;8 Suppl 7:S30-S42. [DOI] [PubMed] [Google Scholar]
- 158. Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41 Suppl 2:97-101. [DOI] [PubMed] [Google Scholar]
- 159. Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200-205. [DOI] [PubMed] [Google Scholar]
- 160. Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21:11-23. [DOI] [PubMed] [Google Scholar]
- 161. Incalza MA, D’Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol. 2018;100:1-19. [DOI] [PubMed] [Google Scholar]
- 162. Hurtubise J, McLellan K, Durr K, et al. The different facets of dyslipidemia and hypertension in Atherosclerosis. Curr Atheroscler Rep. 2016;18:82. [DOI] [PubMed] [Google Scholar]
- 163. Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46-56. [DOI] [PubMed] [Google Scholar]
- 164. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Frühbeck G. Vasoactive factors and inflammatory mediators produced in adipose tissue. In: Fantuzzi G, Mazzone T. eds. Adipose Tissue and Adipokines in Health and Disease. Humana Press;2023:63-77. [Google Scholar]
- 166. Nilsson PM, Natali A. Insulin and blood pressure relationships. In: Berbari AE, Mancia G, eds. Blood Pressure Disorders in Diabetes Mellitus. Springer International Publishing;2023:119-128. [Google Scholar]
- 167. Saeedi P, Salpea P, Karuranga S, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020:108086. [DOI] [PubMed] [Google Scholar]
- 168. Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149-1160. [DOI] [PubMed] [Google Scholar]
- 169. Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003;89:1104-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Guyton AC. The relationship of cardiac output and arterial pressure control. Circulation. 1981;64:1079-1088. [DOI] [PubMed] [Google Scholar]
- 171. Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med. 2003;139:824-834. [DOI] [PubMed] [Google Scholar]
- 172. Ali N, Diamond DM, Rice SM. Cardiovascular disease and its association with insulin resistance and cholesterol. In: Noakes TD, Murphy T, Wellington N, et al. eds. Ketogenic. Elsevier;2023:205-236. [Google Scholar]
- 173. Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37:1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]