Abstract
Soybean (Glycine max [L.] Merr.) leaves have been shown to contain three forms of nitrate reductase (NR). Two of the forms, which are present in leaves of wild-type (cv. Williams) plants grown in the absence of NO3−, are termed constitutive and designated c1NR and c2NR. The third form, which is present in NO3−-grown mutant (nr1) plants lacking the constitutive forms, is termed inducible and designated iNR. Samples of c1NR, c2NR, and iNR obtained from appropriately treated plants were analyzed for the presence of partial activities, response to inhibitors, and ability to complement a barley NR which lacks the molybdenum cofactor (MoCo) but is otherwise active.
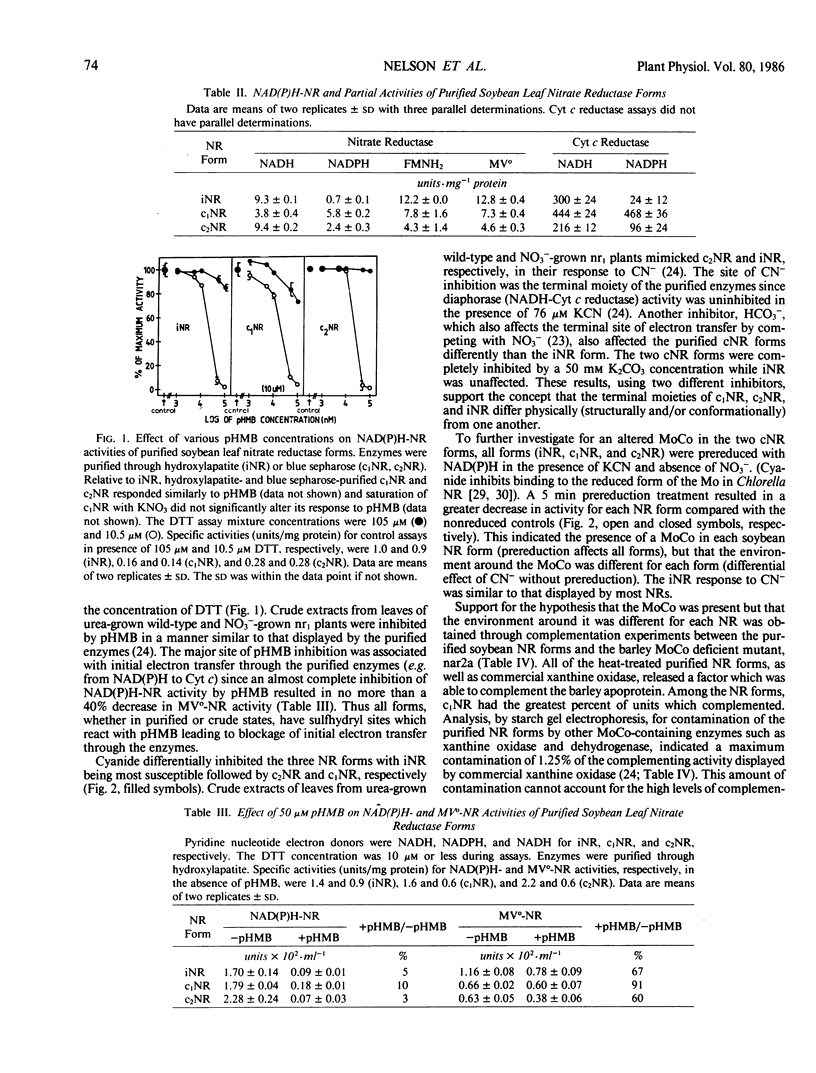
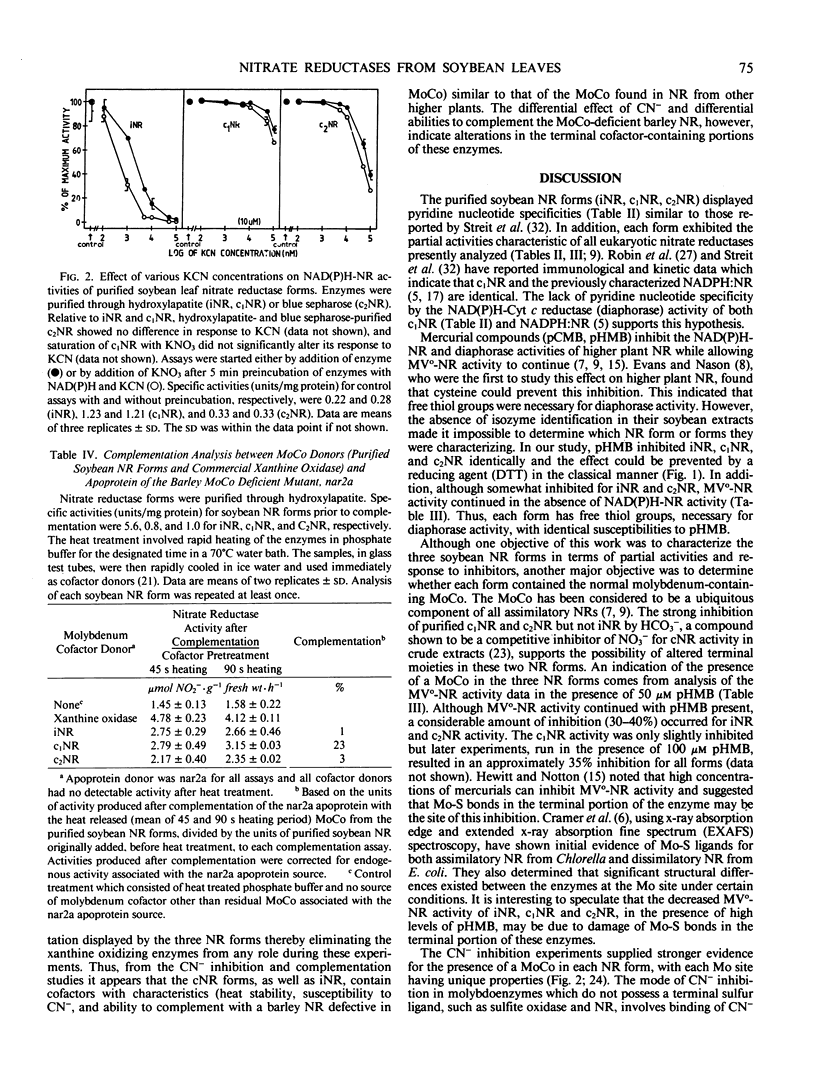
The three forms were similar to most assimilatory NR enzymes in that they (a) exhibited NADH-cytochrome c reductase, reduced flavin mononucleotide-NR, and reduced methyl viologen-NR partial activities; (b) were inhibited by p-hydroxymercuribenzoate at the site of initial electron transport through each enzyme; (c) were more inhibited by CN− in their reduced enzyme state as compared with their oxidized state; and (d) complemented a MoCo-defective NR (e.g. contained cofactors with characteristics similar to the MoCo found in barley NR and commercial xanthine oxidase). However, among themselves, they showed dissimilarities in their response to treatment with HCO3− and CN−, and in their absolute ability to complement the barley NR. The site of effect for these treatments was the terminal cofactor-containing portion of each enzyme. This indicated that, although a terminal cofactor (presumably a MoCo) was present in each form, structural or conformational differences existed in the terminal cofactor-protein complex of each form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aryan A. P., Batt R. G., Wallace W. Reversible Inactivation of Nitrate Reductase by NADH and the Occurrence of Partially Inactive Enzyme in the Wheat Leaf. Plant Physiol. 1983 Mar;71(3):582–587. doi: 10.1104/pp.71.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M. Differential effect of tungsten on the development of endogenous and nitrate-induced nitrate reductase activities in soybean leaves. Plant Physiol. 1982 Jul;70(1):35–38. doi: 10.1104/pp.70.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E., Nicholas J. C. Nitrogen metabolism of soybeans: I. Effect of tungstate on nitrate utilization, nodulation, and growth. Plant Physiol. 1978 Oct;62(4):662–664. doi: 10.1104/pp.62.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Jolly S. O., Campbell W., Tolbert N. E. NADPH- and NADH-nitrate reductases from soybean leaves. Arch Biochem Biophys. 1976 Jun;174(2):431–439. doi: 10.1016/0003-9861(76)90371-4. [DOI] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H., Duke S. O. Differential light induction of nitrate reductases in greening and photobleached soybean seedlings. Plant Physiol. 1983 Sep;73(1):56–60. doi: 10.1104/pp.73.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. S., Ryan S. A., Harper J. E. Soybean mutants lacking constitutive nitrate reductase activity : I. Selection and initial plant characterization. Plant Physiol. 1983 Jun;72(2):503–509. doi: 10.1104/pp.72.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas D. J., Nason A. Role of Molybdenum as a Constituent of Nitrate Reductase from Soybean Leaves. Plant Physiol. 1955 Mar;30(2):135–138. doi: 10.1104/pp.30.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relimpio A. M., Aparicio P. J., Paneque A., Losada M. Specific protection against inhibitors of the NADH-nitrate reductase complex from spinach. FEBS Lett. 1971 Oct 1;17(2):226–230. doi: 10.1016/0014-5793(71)80152-7. [DOI] [PubMed] [Google Scholar]
- Robin P., Streit L., Campbell W. H., Harper J. E. Immunochemical Characterization of Nitrate Reductase Forms from Wild-Type (cv Williams) and nr(1) Mutant Soybean. Plant Physiol. 1985 Jan;77(1):232–236. doi: 10.1104/pp.77.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. A., Nelson R. S., Harper J. E. Soybean Mutants Lacking Constitutive Nitrate Reductase Activity : II. Nitrogen Assimilation, Chlorate Resistance, and Inheritance. Plant Physiol. 1983 Jun;72(2):510–514. doi: 10.1104/pp.72.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Howard W. D., Johnson J. L., Rajagopalan K. V. Electron paramagnetic resonance studies on the molybdenum center of assimilatory NADH:nitrate reductase from Chlorella vulgaris. J Biol Chem. 1984 Jan 25;259(2):849–853. [PubMed] [Google Scholar]
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L. Nitrate reductase-deficient mutants in barley : immunoelectrophoretic characterization. Plant Physiol. 1983 Jan;71(1):145–149. doi: 10.1104/pp.71.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit L., Nelson R. S., Harper J. E. Nitrate Reductases from Wild-Type and nr(1)-Mutant Soybean (Glycine max [L.] Merr.) Leaves : I. Purification, Kinetics, and Physical Properties. Plant Physiol. 1985 May;78(1):80–84. doi: 10.1104/pp.78.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]


