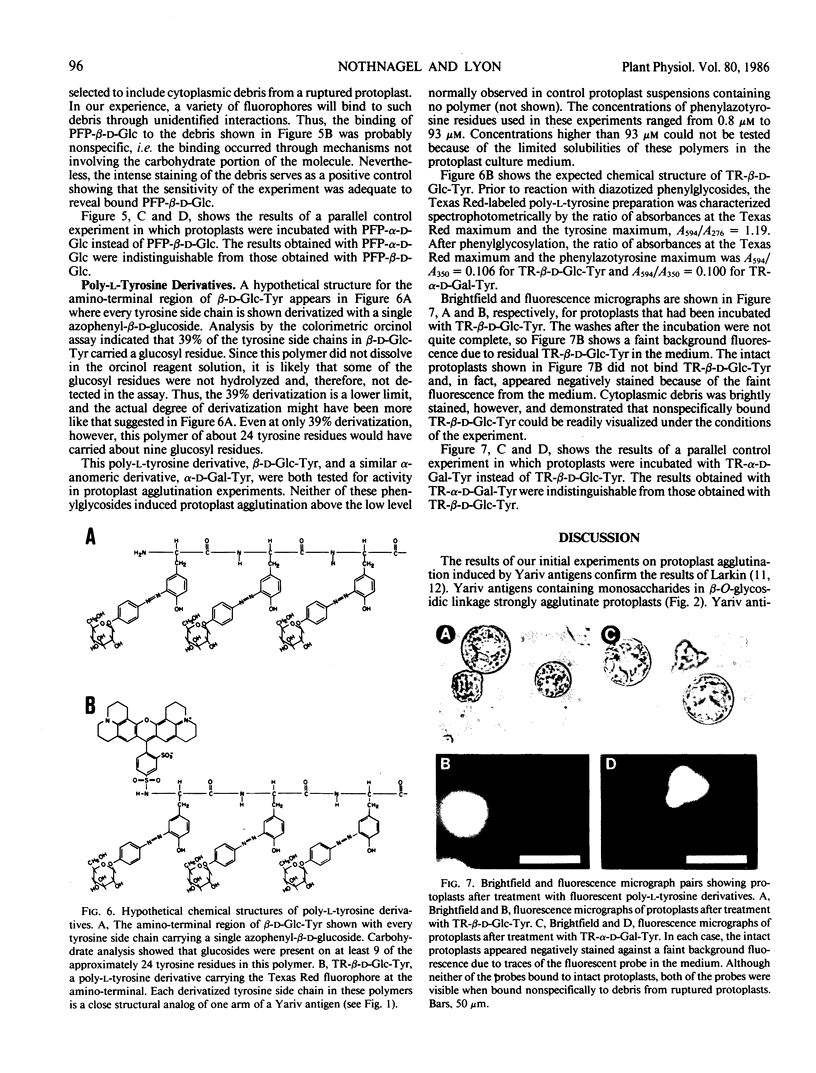
Abstract
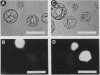
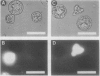
A variety of phenylglycosides have been synthesized and tested for binding to the surface of protoplasts from suspension-cultured cells of “Paul's Scarlet” rose (Rosa sp.). Multivalent phenylglycosides in the form of Yariv antigens (1,3,5,-tri-[p-glycosyloxyphenylazo]-2,4,6,-trihydroxybenzene) agglutinated the protoplasts. Fluorescence-labeled derivatives of other monovalent and polyvalent phenyl-β-glycosides did not bind to the protoplast surface. Agglutination was induced by Yariv antigens only if these probes contained β-anomeric, O-glycosidic linkages. Yariv antigens containing α-anomeric or thio-glycosidic linkages did not agglutinate protoplasts. These same structural features of Yariv antigens were also required for the precipitation of gum arabic-Yariv antigen complexes. The results suggest that plasma membranes of “Paul's Scarlet” rose protoplasts contain arabinogalactan-proteins that interact with phenyl-β-glycosides. The results further show that binding at these plasma membrane sites is not solely dependent upon the carbohydrate portion of single phenylglycosides, but may also require specific spatial orientations of adjacent phenylglycosides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- Clarke A., Gleeson P., Harrison S., Knox R. B. Pollen-stigma interactions: Identification and characterization of surface components with recognition potential. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3358–3362. doi: 10.1073/pnas.76.7.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Larkin P. J. Plant protoplast agglutination and membrane-bound beta-lectins. J Cell Sci. 1977 Aug;26:31–46. doi: 10.1242/jcs.26.1.31. [DOI] [PubMed] [Google Scholar]
- Larkin P. J. Plant protoplast agglutination by artificial carbohydrate antigens. J Cell Sci. 1978 Apr;30:283–292. doi: 10.1242/jcs.30.1.283. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Distribution of fluorescently labeled actin in living sea urchin eggs during early development. J Cell Biol. 1979 Jun;81(3):672–679. doi: 10.1083/jcb.81.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy L., Kimura J., Bing D. H., Parker D. C. Affinity labeling of rabbit antisaccharide antibodies. Biochemistry. 1967 Jul;6(7):1981–1988. doi: 10.1021/bi00859a015. [DOI] [PubMed] [Google Scholar]
- YARIV J., RAPPORT M. M., GRAF L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J. 1962 Nov;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Klis F. M., de Wildt P. J., Hazenberg C. A., Buijs J., Stegwee D. Arabinogalactan Protein from a Crude Cell Organelle Fraction of Phaseolus vulgaris L. Plant Physiol. 1981 Oct;68(4):910–913. doi: 10.1104/pp.68.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]