Abstract
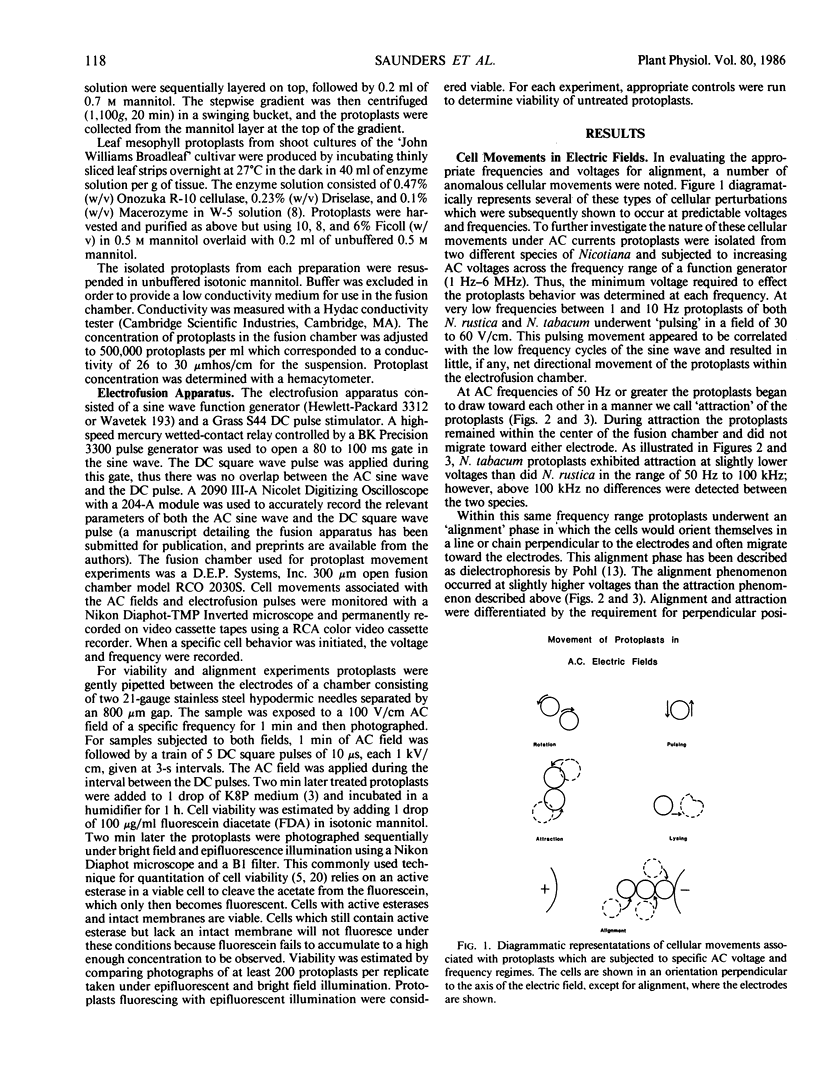
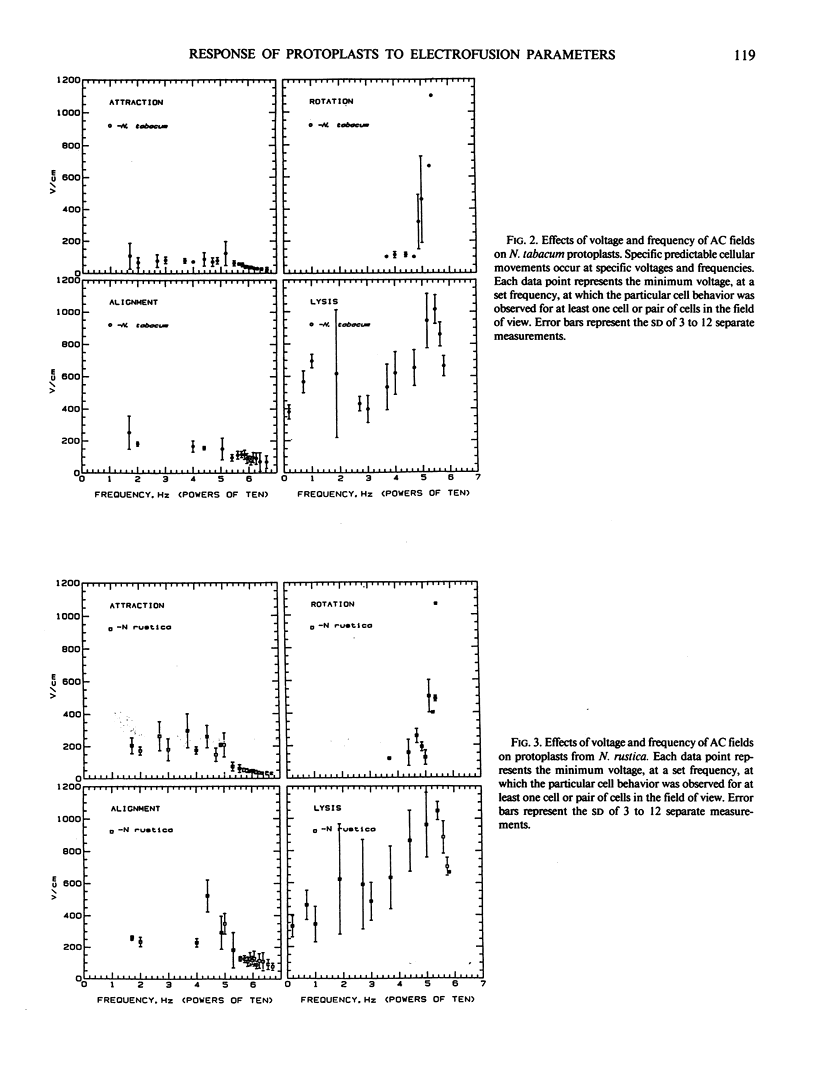
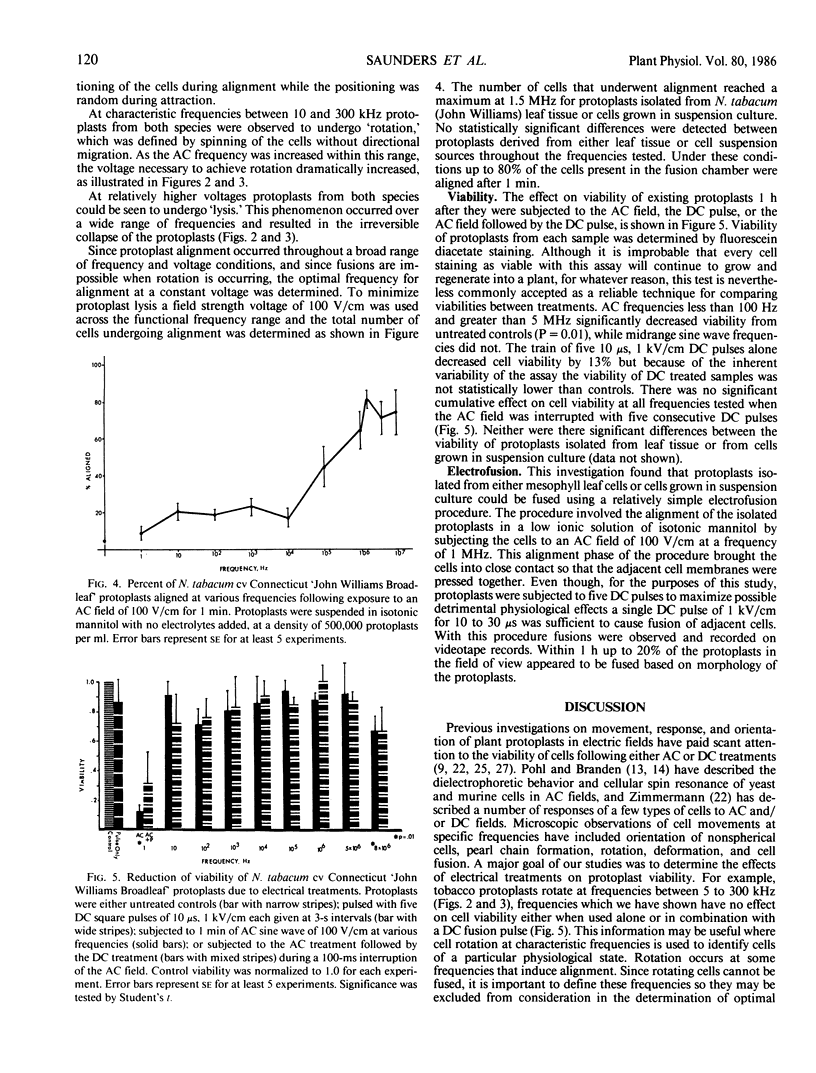
This investigation examines responses of protoplasts in a systematic and quantitative way to the various electrical treatments used to achieve electrofusion and their individual and cumulative effect on protoplast viability. Mesophyll and cell suspension protoplasts from two species of the same genera, Nicotiana tabacum and N. rustica var brasilia were used in these experiments. Optimal frequencies for alignment of tobacco protoplasts were between 500 kilohertz and 2 megahertz at 100 volts per centimeter. Variations in frequency and voltage of the alternating current (AC) field caused predictable movements of protoplasts within an electrofusion chamber. AC frequencies below 10 hertz or above 5 megahertz significantly decreased the viability of protoplasts in the fusion chamber as estimated by fluorescein diacetate staining 1 hour after treatment. Although the direct current (DC) pulse appeared to have a slight detrimental effect on protoplast viability, this effect was not significantly different from untreated control preparations.
Protoplasts from both leaf mesophyll cells and suspension cells were induced to fuse with one or more 10 to 30 microseconds DC square wave pulses of approximately 1 kilovolt per centimeter after the protoplasts had been closely appressed with an AC field.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates G. W., Gaynor J. J., Shekhawat N. S. Fusion of plant protoplasts by electric fields. Plant Physiol. 1983 Aug;72(4):1110–1113. doi: 10.1104/pp.72.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Corn Root Protoplasts: ISOLATION AND GENERAL CHARACTERIZATION OF ION TRANSPORT . Plant Physiol. 1980 Oct;66(4):550–554. doi: 10.1104/pp.66.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D. Characteristics of teratomas regenerated in vitro from octopine-type crown gall. Plant Physiol. 1982 Jan;69(1):37–40. doi: 10.1104/pp.69.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Gillespie J. M. Localization and Substrate Specificity of Glycosidases in Vacuoles of Nicotiana rustica. Plant Physiol. 1984 Dec;76(4):885–888. doi: 10.1104/pp.76.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol. 1974 Dec;54(6):936–944. doi: 10.1104/pp.54.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol. 1982;67(3):165–182. doi: 10.1007/BF01868659. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J., Pilwat G. Rotation of cells in an alternating electric field: the occurrence of a resonance frequency. Z Naturforsch C. 1981 Jan-Feb;36(1-2):173–177. doi: 10.1515/znc-1981-1-229. [DOI] [PubMed] [Google Scholar]


