Abstract
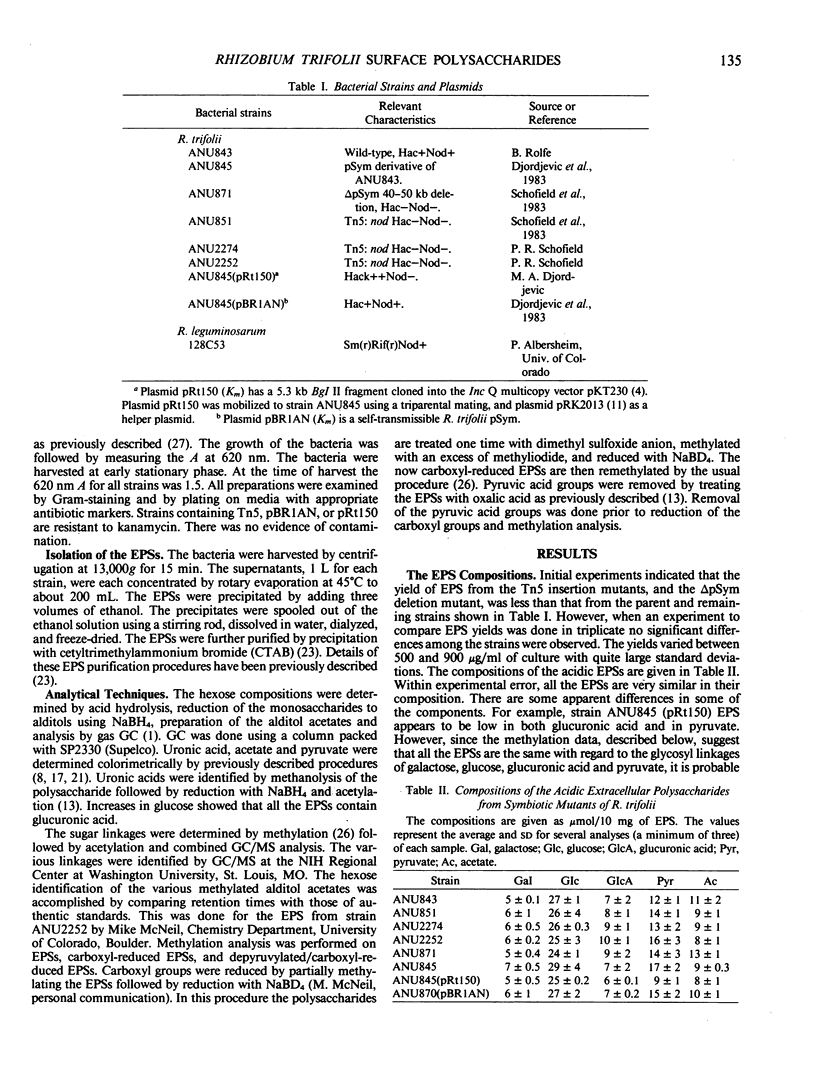
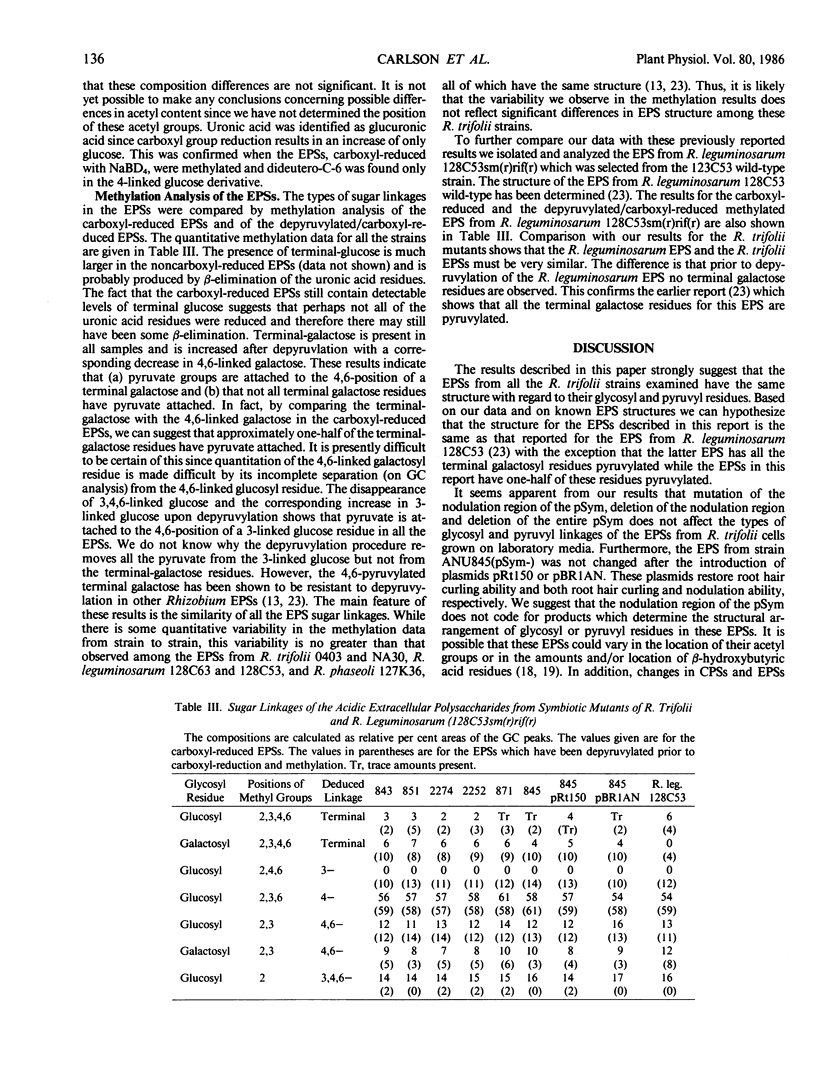
The structures of the acidic extracellular polysaccharides (EPSs) from several R. trifolii mutants were compared by examining their compositions and their sugar linkages as determined by methylation analysis. These mutant strains were derived from the wild-type R. trifolii ANU843 and were unable to induce normal root hair curling (Hac- phenotype) or nodulation response (Nod- phenotype) in clover plants. These strains included several transposon Tn5-induced Nod-mutants, strain ANU871, which possesses a 40 to 50 kilobase deletion of the resident Sym plasmid, and strain ANU845 which is missing the Sym plasmid (pSym-). Strains ANU845(pSym-) containing either plasmid pRt150 or pBR1AN were also used. The recombinant plasmid pRt150 restores only root hair curling capacity to ANU845 while plasmid pBR1AN (an R. trifolii pSym) restores both root hair curling and nodulation capacity to this strain. Our composition and methylation results show that the EPSs from all these strains have the same glycosyl and pyruvyl linkages. Thus we suggest that neither the nod genes involved in root hair curling nor the entire pSym encodes for the arrangement of glycosyl or pyruvyl residues in these EPSs. Whether or not the nod genes dictate the location of acetyl or β-hydroxybutyrate substituent groups remains to be determined.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Shine J., Rolfe B. G. Sym plasmid transfer to various symbiotic mutants of Rhizobium trifolii, R. leguminosarum, and R. meliloti. J Bacteriol. 1983 Dec;156(3):1035–1045. doi: 10.1128/jb.156.3.1035-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Abe M., Sherwood J. E., Dazzo F. B. Bacteriophage-induced acidic heteropolysaccharide lyases that convert the acidic heteropolysaccharides of Rhizobium trifolii into oligosaccharide units. J Bacteriol. 1984 Nov;160(2):510–516. doi: 10.1128/jb.160.2.510-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B., Ljunggren H., Lönngren J., Rudén U., Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of Rhizobium meliloti by sequential degradation. J Am Chem Soc. 1977 May 25;99(11):3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Yoshida T., Tanegashima C., Tanaka S. Improved direct method for determination of keto acids by 2,4-dinitrophenylhydrazine. Anal Biochem. 1971 Oct;43(2):349–356. doi: 10.1016/0003-2697(71)90263-6. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


