Abstract
Plasma membrane was isolated in a uniform population and with a high purity from chilling-sensitive etiolated young seedlings of Vigna radiata (mung bean) utilizing an aqueous two polymer phase separation system and subsequent sucrose density gradient. The isolated plasma membrane was associated with vanadate-sensitive and KNO3-insensitive ATPase. The ATPase has high specificities both for substrate and Mg2+ ion with optimum pH at 6.5. It was slightly stimulated by monovalent anions, especially Cl−. Proton ionophores such as gramicidin D and carbonyl cyanide p-trifluoromethoxyphenylhydrazone did not stimulate the enzyme activity. The ATPase is apparently latent and highly stimulated by the addition of detergents such as Triton X-100. A maximum stimulation was achieved by the addition of 0.02% Triton X-100. After treatment with proteinase K in an isotonic buffer solution, the enzyme activity was less affected, whereas the peptides were specifically digested. Based on these facts, the isolated plasma membrane vesicles appear to be tightly sealed and in a right-side-out orientation. The plasma membrane ATPase had two inflection points at higher (18.9°C) and lower (6.7°C) temperatures on the Arrhenius plots of the activity. The lower inflection temperature apparently coincided with that of the anisotropy parameter of embedded 1,6-diphenyl-1,3,5-hexatriene, indicating that the membrane bound ATPase activity was affected by a phase transition of membrane lipids and/or temperature-dependent conformational changes in the enzyme molecules per se. Considering the fact that the plant material used here is highly sensitive to chilling temperatures and injured severely by exposure to temperatures below 5°C for a relatively short period, the thermotropic properties of membrane molecules are considered to be involved in the mechanism of chilling injury.
Full text
PDF

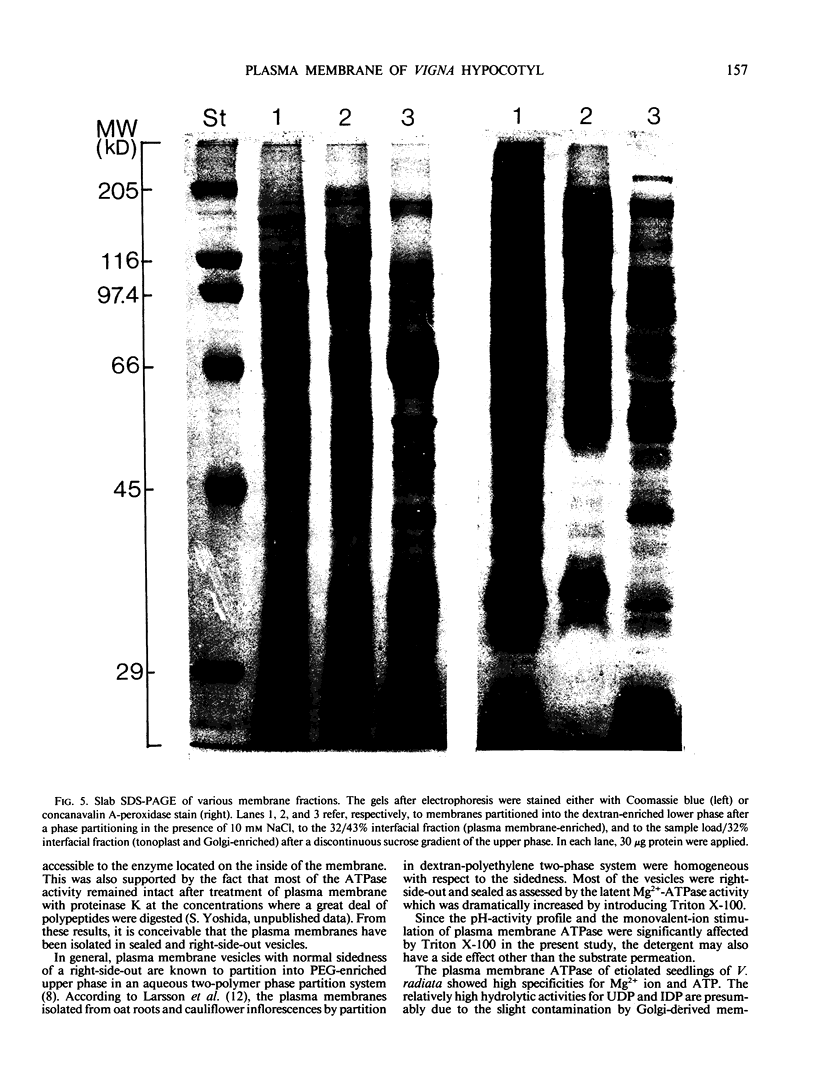
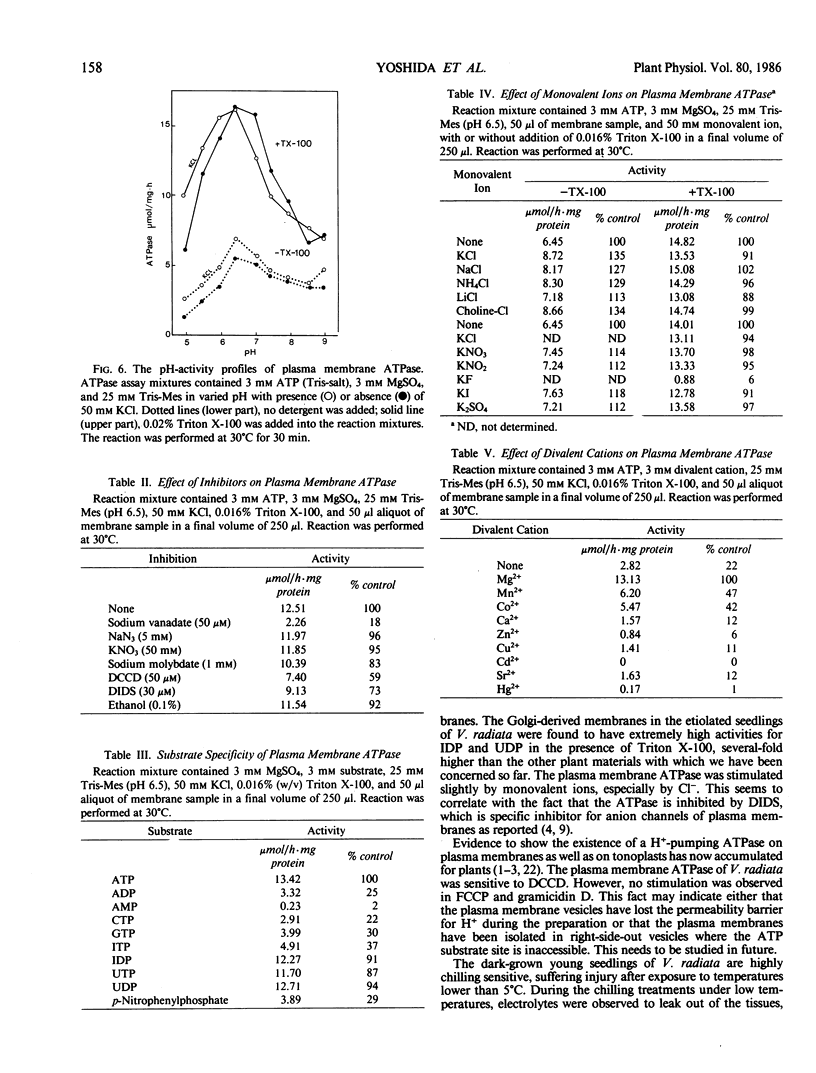
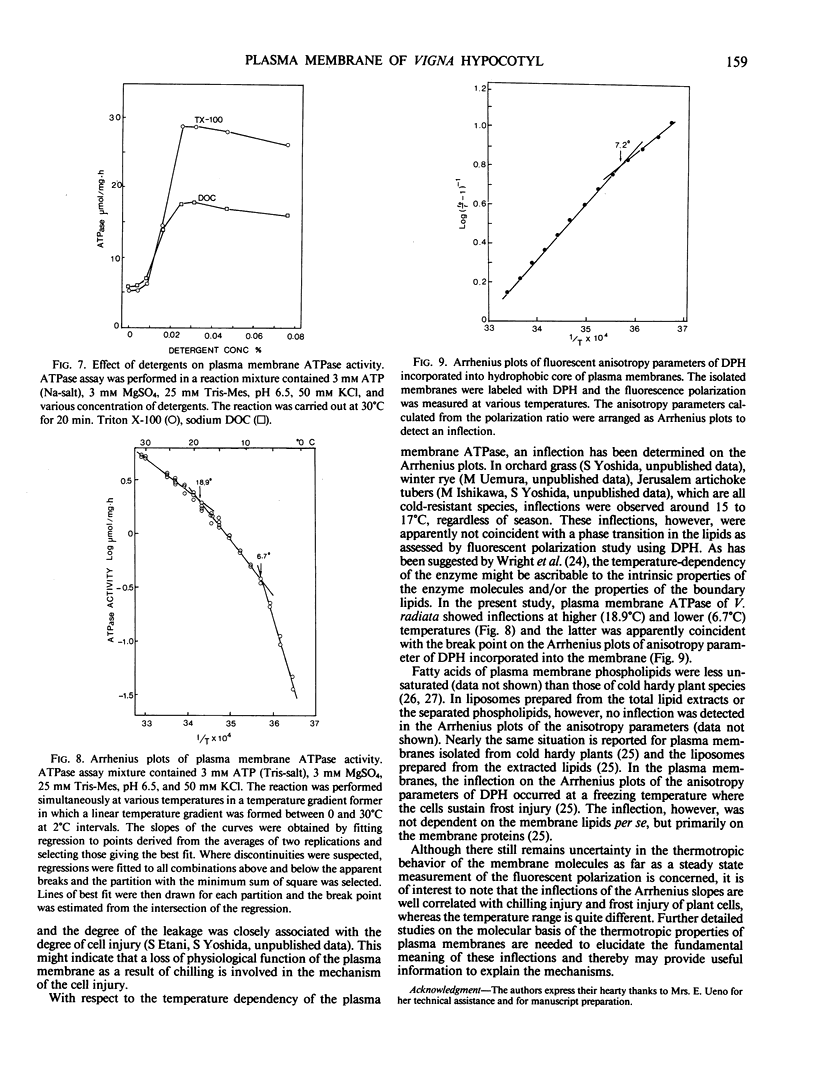

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-Sensitive, H-Pumping ATPase of Oat Roots : Direct Effects of Cl, NO(3), and a Disulfonic Stilbene. Plant Physiol. 1984 Oct;76(2):490–497. doi: 10.1104/pp.76.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hallberg M., Larsson C. Compartmentation and export of 14CO2 fixation products in mesophyll protoplasts from the C4-plant Digitaria sanguinalis. Arch Biochem Biophys. 1981 Apr 15;208(1):121–130. doi: 10.1016/0003-9861(81)90130-2. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Franceschi V. R., Lucas W. J. Plasmalemma Chloride Transport in Chara corallina: Inhibition by 4,4'-Diisothiocyano-2,2'-Disulfonic Acid Stilbene. Plant Physiol. 1982 Nov;70(5):1327–1334. doi: 10.1104/pp.70.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Debey P., Sabatini D. D. Selective release of content from microsomal vesicles without membrane disassembly. I. Permeability changes induced by low detergent concentrations. J Cell Biol. 1973 Aug;58(2):436–462. doi: 10.1083/jcb.58.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. Electrogenic transport of protons driven by the plasma membrane ATPase in membrane vesicles from radish : biochemical characterization. Plant Physiol. 1985 Jan;77(1):200–205. doi: 10.1104/pp.77.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Sarinana F. O. The staining of sciatic nerve glycoproteins on polyacrylamide gels with concanavalin A-peroxidase. Anal Biochem. 1975 Nov;69(1):320–322. doi: 10.1016/0003-2697(75)90597-7. [DOI] [PubMed] [Google Scholar]
- Wright L. C., McMurchie E. J., Pomeroy M. K., Raison J. K. Thermal behavior and lipid composition of cauliflower plasma membranes in relation to ATPase activity and chilling sensitivity. Plant Physiol. 1982 Jun;69(6):1356–1360. doi: 10.1104/pp.69.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Studies on Freezing Injury of Plant Cells: I. Relation between Thermotropic Properties of Isolated Plasma Membrane Vesicles and Freezing Injury. Plant Physiol. 1984 May;75(1):38–42. doi: 10.1104/pp.75.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]