Abstract
Endogenous neurosteroids and synthetic neuroactive steroids (NAS) are important targets for therapeutic development in neuropsychiatric disorders. These steroids modulate major signaling systems in the brain and intracellular processes including inflammation, cellular stress and autophagy. In this review, we describe studies performed using unnatural enantiomers of key neurosteroids, which are physiochemically identical to their natural counterparts except for rotation of polarized light. These studies led to insights in how NAS interact with receptors, ion channels and intracellular sites of action. Certain effects of NAS show high enantioselectivity, consistent with actions in chiral environments and likely direct interactions with signaling proteins. Other effects show no enantioselectivity and even reverse enantioselectivity. The spectrum of effects of NAS enantiomers raises the possibility that these agents, once considered only as tools for preclinical studies, have therapeutic potential that complements and in some cases may exceed their natural counterparts. Here we review studies of NAS enantiomers from the perspective of their potential development as novel neurotherapeutics.
Keywords: Allopregnanolone, Pregnanolone, Pregnenolone sulfate, Neuroprotection, Neuroinflammation, GABAA receptors, NMDA receptors, Calcium channels
1. Introduction
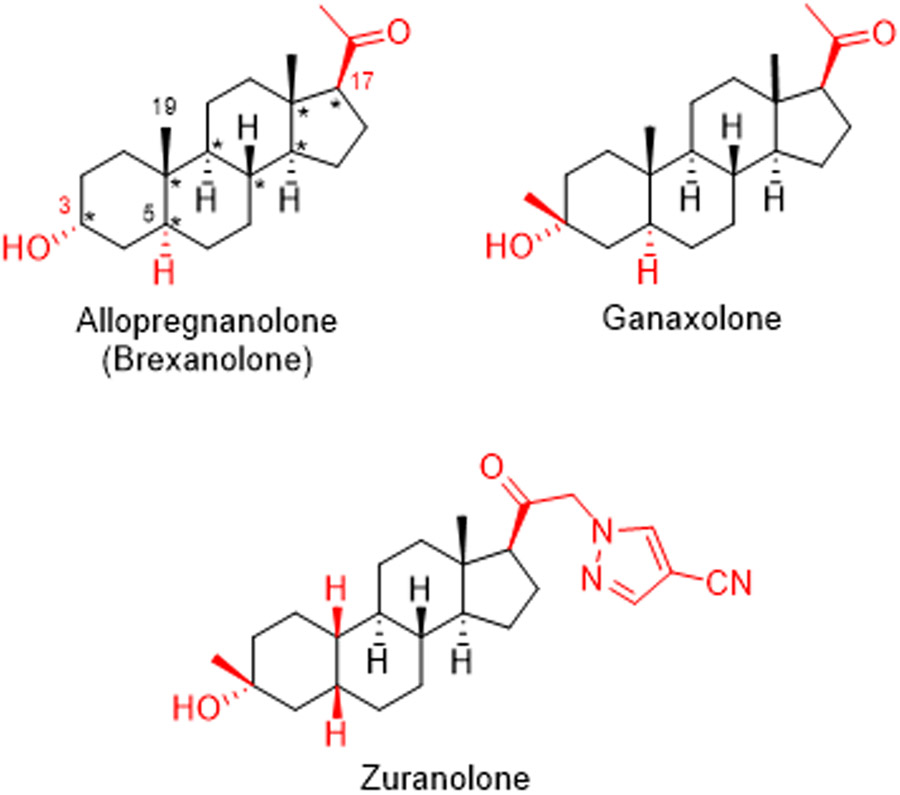
There is now considerable interest in developing neuroactive steroids (NAS) as novel treatments for a variety of neuropsychiatric illnesses. The past three years have witnessed the approval of two NAS by the US Food and Drug Administration – brexanolone in 2019 for treatment of postpartum depression (PPD), and ganaxolone in 2022 for treatment of seizures in the context of a neurodevelopmental disorder caused by cyclin-dependent kinase-like 5 deficiency (Meltzer-Brody and Kanes, 2020; Lamb, 2022). A third NAS, zuranolone, is in late phase clinical trials for use in major depressive disorder (MDD) and PPD (Gunduz-Bruce et al., 2019; Deligiannidis et al., 2021) (Fig. 1). NAS are also under development and in clinical trials for other indications including epilepsy, neurodegenerative illnesses, essential tremor and anesthesia, among further potential uses (Belelli et al., 2020; Goodchild et al., 2020; Johansson et al., 2016; Mitchell et al., 2008; Raikes et al., 2021; Reddy and Estes, 2016; Zorumski et al., 2019).
Fig.1.
Neuroactive steroids in clinical use or development. The eight chiral centers in allopregnanolone (brexanolone) are marked with asterisks. By convention, dashed and solid wedges designate the α and β configurations of substituents at chiral stereocenters. Important structural features described in the text are highlighted in red font.
The approved NAS are potent and highly effective positive allosteric modulators (PAMs) of Δ-aminobutyric acid A receptors (GABAARs), the predominant class of receptors mediating both phasic (synaptic) and tonic (extrasynaptic) inhibition in the brain (Zorumski et al., 2013). The steroids presently approved and in development have similar, though not identical effects on GABAARs and differ in chemical structure. Brexanolone is a formulation of the endogenous neurosteroid, allopregnanolone (AlloP, 3α-hydroxy-5α-pregnan-20-one) in a cyclodextrin for intravenous infusion (Meltzer-Brody and Kanes, 2020). The other two steroids in advanced development are orally active agents. Ganaxolone is a 3α-hydroxyl, 5α-reduced steroid that also features a 3β-methyl group that improves oral bioavailability (Lamb, 2022). Similarly, zuranolone has 3α-hydroxyl and 3β-methyl substitutions but is also 5β-reduced without a C-19 methyl group, and features a more complex substitution at C-17 of the steroid D-ring that improves bioavailability (Martinez Botella et al., 2017; Althaus et al., 2020) (Fig. 1).
Detailed structure-activity studies of NAS indicate that there are two critical portions of the molecule for GABAAR PAM activity (Covey et al., 2001; Zorumski et al., 2013). These include a hydrogen bond donor in the 3α configuration (usually a hydroxyl group) and a C-17β hydrogen bond acceptor (a methyl ketone in the case of brexanolone and ganaxolone). Configuration at C-5 is less critical, and both 5α- (brex-anolone and ganaxolone) and 5β-reduced (zuranolone) steroids are effective at enhancing activity at GABAARs and have progressed in human clinical trials.
The endogenous neurosteroid, AlloP, has eight chiral centers and a key finding made over 25 years ago is that activity of AlloP and structural analogues are highly enantioselective for PAM activity at GABAARs, with EC50 values differing by over an order of magnitude between the natural and unnatural steroids (Wittmer et al., 1996). 5β-Reduced neurosteroids (e.g. pregnanolone) also show enantioselectivity at GABAARs, but potency separation between enantiomers is only about 3–4-fold (Covey et al., 2000, 2001; Evers et al., 2000). These enantiomers represented a major advance in understanding NAS as GABAAR modulators and indicated strongly that these neurosteroids act in chiral environments likely on the receptors. This concept was borne out by later site-directed mutagenesis, photoaffinity labeling and structural studies (Hosie et al., 2006; Chen et al., 2019; Sun et al., 2023). In contrast, accumulation of NAS in membranes and intracellular compartments shows no enantioselectivity (Chisari et al., 2009, 2010; Jiang et al., 2016). However, the lack of activity at GABAARs and further study of enantiomeric steroids has resulted in identification of unexpected actions of these agents – actions that suggest strongly that unnatural NAS analogues may have unique therapeutic potential that can both complement and differ from the natural steroids. In this paper, we review the evolution and current state of NAS enantiomers, and highlight important effects of these agents that have therapeutic potential in neuropsychiatric illnesses.
2. Literature search
To date and to our knowledge, almost all unnatural NAS enantiomers have been synthesized in the laboratory of the first author of this paper and have been disseminated to other laboratories for independent testing. Thus, this review emphasizes work done with these compounds in laboratories within and outside the United States over the past 30 years. We also conducted a literature search using PubMed and Chemical Abstracts with search terms that included neurosteroid enantiomers, allopregnanolone, pregnanolone, androgens, estrogens and sulfated steroids. We refined the search returns to emphasize work done with unnatural enantiomers. Broad effects of natural neurosteroids have been reviewed elsewhere (Belelli et al., 2020; Mitchell et al., 2008; Reddy et al., 2016; Zorumski et al., 2013, 2019).
3. Neurosteroids & neurosteroid enantiomers
3.1. Definitions and chemistry of enantiomers
The term “neurosteroid” was first used by Etienne Baulieu and colleagues in the early 1980′s to describe the finding that dehydroepiandrosterone sulfate is synthesized in situ in rat brain (Baulieu, 1981; Corpechot et al., 1981). The term is now widely used for steroids made in the brain that have rapid non-genomic effects on neuronal activity. This definition should not be interpreted to mean that neurosteroids made in the brain are not also made in other organs of the body. For example, the adrenal gland, testes and ovaries make steroid hormones that are also synthesized in brain; and progesterone, testosterone and 17β-estradiol, which are referred to as sex hormones in the endocrine literature, are widely referred to as neurosteroids in the neuroscience literature. Further, the periphery may serve as a major endogenous source of neurosteroids that are also synthesized in the brain. Analogues of neurosteroids are referred to as “neuroactive steroids” (NAS), a term coined by Steven Paul and Robert Purdy in the early 1990′s (Paul and Purdy, 1992). The term “NAS” has broader definition than “neurosteroid” and includes neurosteroids made in brain, endogenous steroids made in the body that impact brain function, and synthetic steroids that modulate brain activity.
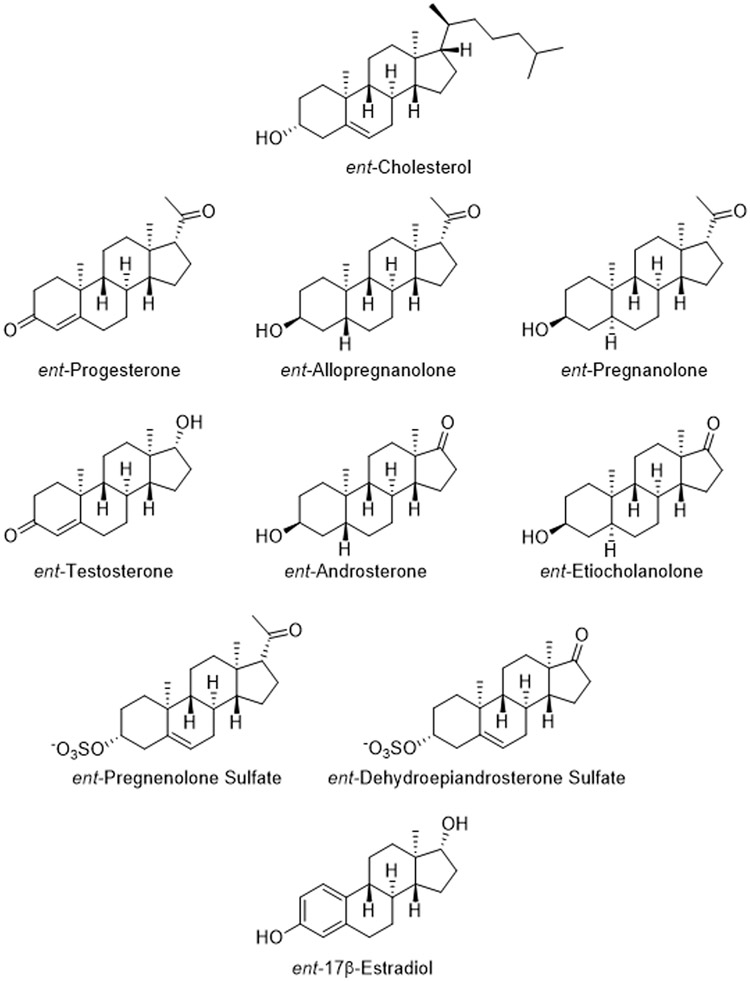
The mirror images (enantiomers) of naturally occurring steroids are defined as ent-steroids. Enantiomeric steroids are sometimes mistaken for diastereomeric steroids. In molecules like steroids which have more than one chiral center inverting one or more, but not all, chiral centers produces a diastereomer, which is not a mirror image (i.e. not an enantiomer). For example, changing the configuration of the 3-hydroxyl group of AlloP from the alpha to the beta configuration yields epiallopregnanolone (also named as isoallopregnanolone), a diasteromer, not an enantiomer of AlloP. This distinction is important because only enantiomers, and not diastereomers, have identical physiochemical properties (except for rotation of polarized light). Thus conclusions based on diastereomers have the major limitation that these molecules have different physical properties that could account for any differences in actions. Biosynthetic pathways for the conversion of squalene, the precursor to steroids, to ent-steroids are unknown as are biosynthetic pathways for the interconversion of a steroid to its corresponding ent-steroid. Accordingly, ent-steroids are made by chemical synthesis. A review by Biellmann (2003) describes synthetic methods for their preparation. More recent synthetic methods also exist (Jastrzebska and Covey, 2022), but are not reviewed here.
Because ent-neurosteroids are mirror images of the naturally occurring neurosteroids (Covey et al., 2001), all of the stereocenters, indicated by wedges (β configuration) and dashed wedges (α configuration) in Fig. 2, are the opposite of the stereocenters in their corresponding neurosteroids. Fig. 2 shows the structures of the enantiomers of cholesterol and of the most studied neurosteroids. Cholesterol fulfills part of the definition of “neurosteroid” because the brain makes its own cholesterol; peripheral cholesterol bound to lipoproteins such as LDL does not cross the blood brain barrier (Bjorkhem and Meaney, 2004). Although not reviewed here, ent-cholesterol has been used in many different studies (for early reviews see Westover and Covey, 2004; Covey, 2009). The role of cholesterol and its metabolites in the brain is also currently of great interest in Alzheimer’s and other neurodegenerative diseases (Kacher et al., 2022; Rudajev and Novotny, 2022).
Fig. 2.
Structures of enantiomeric steroids. Stereocenters are denoted by solid (β configuration) or dashed (α configuration) wedges and are the opposite of the stereocenters in naturally occurring steroids. Comparison of the structures ent-allopregnanolone and naturally occurring allopregnanolone (Fig. 1) shows the opposite configuration of all eight chiral centers.
3.2. Uses of enantiomers
Neurosteroid enantiomers were initially prepared to investigate mechanisms underlying the anesthetic actions of AlloP. In 1986, it was established that AlloP potentiated the actions of GABA at GABAA receptors (Majewska et al., 1986). Potentiation of this receptor class contributes strongly to the mechanism of action for many general anesthetics in clinical use. Anesthetics are hydrophobic molecules and the long-standing Meyer-Overton hypothesis correlated anesthetic potency with their accumulation in membranes such that changes in membrane properties indirectly affected ion channel function. Specific binding sites for anesthetics on ion channels are not a premise of this hypothesis. Although it is now clear that there are specific binding sites for AlloP on GABAA receptors, this was not the case prior to 2006 when site-directed mutagenesis studies first unambiguously identified these sites (Hosie et al., 2006). Subsequent studies using x-ray crystallography and photoaffinity labeling methods have highlighted the complexity of NAS interactions with GABAARs including multiple likely sites of receptor interaction (Chen et al., 2012; 2019; Laverty et al., 2017; Miller et al., 2017; Sugasawa et al., 2020, Sun et al., 2023). Earlier in the mid-1990 s, a medicinal chemical approach was taken to provide evidence that AlloP’s anesthetic actions resulted from its direct binding to GABAA receptors, not indirect actions caused by changes in membrane properties (Wittmer et al., 1996). Thus, ent-AlloP was synthesized and studied with the rationale being that a binding site on a receptor would likely distinguish AlloP from its enantiomer, whereas the membrane perturbation effects of AlloP and its enantiomer, which have identical physiochemical properties, would be equivalent leading to no difference in their actions. Because ent-AlloP was not a potent modulator of GABAA receptors, this provided the first evidence for steroid binding sites on this class of receptors. Furthermore, these early studies provided evidence that anesthetic effects of AlloP and analogues in tadpoles and mice also show strong enantioselectivity (Wittmer et al., 1996). These initial results also provided the impetus to study other ent-neurosteroids and propelled investigations into the reasons for the observed enantioselectivity at GABAA receptors.
Since the initial report of enantioselective actions at GABAARs, work with stereoisomers and enantiomers has progressed and provided important insights into how NAS likely align with GABAARs to modulate function (Covey et al., 2000). In this regard, the substituent at the 17-position on the steroid D-ring is a major determinant of the stereoselectivity found for both AlloP and pregnanolone, the 5β-reduced analogue of AlloP (Krishnan et al., 2012). Changing the acetyl group at C-17 to a ketone group produces the androgen class steroids, androsterone and etiocholanolone, respectively. Interestingly, this structural change leads to a reversal of enantioselective action. Both ent-androsterone and ent-ethiocholanolone (Fig. 2) are more active GABAAR PAMs than their corresponding natural NAS (Katona et al., 2008). Additional studies of structural modifications of substituents on the steroid D-ring have led to the discovery of ent-steroids with potent anesthetic and anticonvulsant actions (Krishnan et al., 2012; Qian et al., 2014; Zolkowski et al., 2014). Finally, ent-steroids that are weak or inactive modulators of what is generally considered to be the primary targets for their mechanism of action are powerful tools for identifying unexpected targets and pathways affected by neuroactive steroids. The potential clinical uses for ent-neuroactive steroids are discussed in the remaining sections of the review under the headings of their corresponding natural neurosteroids.
4. Progesterone
4.1. Effects of progesterone in the nervous system
Progesterone (PROG) is an important neurosteroid that is synthesized in brain from cholesterol, where it serves as both a modulator of certain brain functions and as a precursor to multiple other neurosteroids including AlloP (Belelli and Lambert, 2005; Belelli et al., 2020; Liang and Rasmussen, 2018). PROG itself has several well-known actions in the brain including direct effects on intracellular PROG receptors (PRs) that regulate gene transcription (Ghoumari et al., 2020; Gonzalez et al., 2020). Additionally, PROG can act at several other receptors including receptors expressed on the cell surface of neurons and other cells (Wendler and Wehling, 2022). These receptors include a class of G-protein coupled receptors (GPCRs) called membrane progesterone receptors (mPRs) that activate intracellular signaling pathways akin to other GPCRs and have neuroprotective effects (Thomas and Pang, 2020), and PGRMC1 (PROG receptor membrane component 1), receptors that activate cytochrome P450 enzymes, affect lipid metabolism and promote cell survival (Bassani et al., 2022; Ghoumari et al., 2020; Gonzalez et al., 2020). PROG was recently shown to be a PAM of the voltage-gated potassium channel Kir7.1 in epithelial cells of the choroid plexus (Bjorkgren et al., 2022). While it is unclear whether PROG directly modulates GABAARs, it is certain that some of its metabolites (e. g. AlloP and others) are highly effective and direct acting PAMs. Also, activation of mPRs can modulate expression and phosphorylation of GABAAR subunits (Parakala et al., 2019; Vien et al., 2022) to alter GABAergic inhibition. Furthermore, PROG (and AlloP) withdrawal upregulates expression of certain GABAAR subunits (α4 in particular) and this contributes to altered inhibition in the hippocampus and an anxiety-like behavioral phenotype (Gulinello et al., 2002; Smith et al., 2013). PROG can also modulate neuronal excitability via changes in expression of glutamate receptors and other signaling proteins (Kapur and Joshi, 2021).
Among the major effects of PROG in brain is its ability to serve as a neuroprotectant in a wide range of neurodegenerative conditions (Gonzalez et al., 2020), including animal models of traumatic brain injury (TBI) where PROG (and its downstream product AlloP) dampen neuronal death and improve learning and memory (Galani et al., 2001; Shear et al., 2002). Additionally PROG (and AlloP) dampen production of proinflammatory cytokines and decrease cerebral swelling along with promoting functional recovery from injury (VanLandingham et al., 2006). Mechanisms involved in neuroprotection by PROG are not completely understood but all three PROG receptors described above have been implicated in the beneficial effects (Bassani et al., 2022; Cao et al., 2021; Ghoumari et al., 2020; Gonzalez et al., 2020) and PROG can upregulate antioxidant signaling and the cellular process of autophagy (Ghandiri et al., 2019; Kim et al., 2012, 2013). One of the challenges in understanding neuroprotective mechanisms of PROG is its metabolism to other active neurosteroids, including AlloP, a powerful GABAAR positive allosteric modulator (PAM) and known neuroprotectant (Zorumski et al., 2019).
4.2. Effects of ent-progesterone
The enantiomer of PROG (ent-PROG) has not been studied extensively but has several important properties (Auchus et al., 2003). ent-PROG binds nuclear PRs with moderate affinity and appears to inhibit binding of PROG competitively (VanLandingham et al., 2006). In COS cells, however, ent-PROG does not activate PR-mediated gene transcription (VanLandingham et al., 2006). Early studies showed that ent-PROG competitively inhibits CYP450c17 and CYP450c21, enzymes that metabolize PROG in the pathway leading to corticosteroids; these two P450 enzymes do not hydroxylate ent-PROG (Auchus et al., 2003). These results indicate that there is enantioselectivity in at least some of PROG’s actions, although it is presently unknown whether other PROG receptors including mPRs and PGRMC1 show similar enantioselectivity.
Intriguingly, in a well-characterized in vivo rat model of TBI, ent-PROG showed substantial neuroprotection at the same dose (16 mg/kg) at which PROG was effective. Effects of both enantiomers resulted in marked decreases in brain edema, reactive gliosis and production of proinflammatory cytokines, along with increases in generation of endogenous antioxidants (VanLandingham et al., 2006). Mechanisms and pathways underlying these effects of ent-PROG are unlikely to involve classical intracellular PRs, but remain unknown.
Results in preclinical studies of PROG led to human clinical trials in TBI, but, to date, those trials have not been successful (Lu et al., 2016; Zeng et al., 2015). Numerous factors could contribute to the failure of these TBI trials, not the least of which would be metabolism of PROG and uncertainties about concentrations of the agent achieved in critical brain regions. Given protective effects of ent-PROG observed in rodent studies, it is possible that this agent could be developed for therapeutic purposes in TBI and perhaps other conditions associated with neurodegeneration and cognitive impairment (VanLandingham et al., 2013). At present little is known about pharmacological properties of ent-PROG.
5. 5α- and 5β-reduced pregnanes
5.1. Effects of pregnane steroids in the nervous system
The prototype for this class of neurosteroids is AlloP, a 3α-hydroxy, 5α-reduced pregnane that is a metabolite of PROG and a potent and effective PAM at GABAARs. This steroid is synthesized endogenously in brain in multiple cell types including excitatory (glutamatergic) neurons in response to certain drugs and acute cellular stressors (Agis-Balboa et al., 2006; Tokuda et al., 2010, 2011; Zorumski and Izumi, 2012). In addition to powerfully modulating neuronal excitability and brain network function via GABA-enhancing effects, AlloP also has sedative/anesthetic, analgesic, anti-inflammatory and neuroprotective effects that make it and its derivatives attractive candidates as possible therapeutics for multiple neuropsychiatric illnesses (Kim et al., 2012; Belelli et al., 2020; Zorumski et al., 2019). Some of these effects (sedation and anticonvulsant effects) are consistent with GABAergic actions, while other effects are less clearly GABA-related.
5.2. Effects of ent-pregnanes
As described above, the enantiomer of AlloP (ent-AlloP) was synthesized in the mid-1990′s (Wittmer et al., 1996; Hu et al., 1997; Covey et al., 2000; Covey, 2009), and its effects and those of structural analogues have been examined in multiple pre-clinical studies. Initial efforts centered on understanding whether effects of AlloP on GABAARs show enantioselectivity as could be expected if this neurosteroid acts in the defined chiral environment of a receptor binding pocket. These early studies showing high degrees of enantioselectivity provided strong support for 3α-hydroxyl, 5α-reduced NAS potentiation and direct gating of GABAARs, with enantiomers differing by at least an order of magnitude in effects on GABA currents and displacement of the non-competitive antagonist, TBPS, in binding assays (Table 1). Similar high enantioselectivity was observed in anesthetic assays in tadpoles and mice (Wittmer et al., 1996). Enantioselectivity also extended to a class of tricyclic AlloP analogues called benz[e]indenes, which have an open A-ring (Rodgers-Neame et al., 1992; Zorumski et al., 1996, 1998).
Table 1.
NAS Enantiomers & TBPS Binding.
| Neurosteroid | Natural Neurosteroid TBPS IC50 (μM) |
Enantiomeric Neurosteroid TBPS IC50 (μM) |
Reference |
|---|---|---|---|
| Allopregnanolone | 0.074 ± 0.007 | 1.91 ± 0.27 | Katona et al. 2008 |
| Alfaxalone | 0.23 ± 0.02 | 5.15 ± 0.60 | Unpublished |
| THDOC | 0.039 ± 0.003 | > 10 | Unpublished |
| Pregnanolone | 0.071 ± 0.018 | 0.28 ± 0.03 | Katona et al. 2008 |
| Androsterone | 0.41 ± 0.13 | 0.31 ± 0.04 | Katona et al. 2008 |
| Etiocholanolone | 4.15 ± 1.00 | 0.38 ± 0.06 | Katona et al. 2008 |
The table displays IC50 values for the ability of NAS enantiomers to displace TBPS binding from rat brain membranes. Abbreviations: Alfaxalone (3α-Hy-droxy-5α-pregnane-11,20-dione); THDOC (3α,21-dihydroxy-5α-pregnan-20-one).
In contrast to AlloP, the enantiomer of pregnanolone (a 3α-hydroxy, 5β-reduced analogue of AlloP) showed much lower (about 3-fold) enantioselectivity in assays of GABAAR activity, TBPS binding and anesthesia (Table 1) (Covey et al., 2000, 2001). The reason for the decreased enantioselectivity of pregnanolone versus AlloP initially suggested in binding sites for these steroids on GABAARs. Possibly consistent with this interpretation, a novel NAS, 3α,5α-17-phenyl-androst-16-en-3-ol (17PA), has no intrinsic effect on GABAARs alone, but acts as an antagonist and effectively inhibits potentiation and direct channel gating by 5α-reduced NAS PAMS while only weakly altering effects of 5β-reduced PAMs. In contrast to 5α-NAS, 17βA has no effect on barbiturate-induced GABAAR potentiation (Mennerick et al., 2004). 17PA and similar agents are referred to as GABAAR modulating steroid antagonists (GAMSA) (Johansson et al., 2016). Interestingly, pregnanolone, in contrast to AlloP, inhibits rather than potentiates a unique homopentameric subtype of GABA receptors expressing rho-1 subunits that are sometimes called GABAc receptors. However, both the inhibitory effects of pregnanolone and the potentiating effects of AlloP on rho-1 receptors are enantioselective, although at higher concentrations, ent-pregnanolone appears to have the opposite effect of pregnanolone, potentiating rho-1 receptors (Li et al., 2006).
Recent photoaffinity labeling studies have examined the interactions of AlloP, pregnanolone and their enantiomers with recombinant α1β3 GABAARs. These studies found that ent-AlloP binds with low affinity to the β3-α1 intersubunit site and in a pose that is inverted 180° from the binding poses of AlloP, pregnanolone, and ent-pregnanolone (Tateiwa et al., 2023). Interestingly, ent-AlloP interacts similarly to AlloP with intrasubunit binding sites on the α1 and β3 subunits. The markedly reduced ability of ent-AlloP to potentiate GABA-elicited currents was attributed to its interaction with the canonical β3-α1 intersubunit NAS binding site.
AlloP also inhibits T-type (low voltage activated) calcium channels in rodent dorsal root ganglion neurons and in recombinant channels. Akin to what was observed with GABAARs, inhibition of T-channels by AlloP and a 5α-reduced structural analogue also exhibited significant enantioselectivity with an order of magnitude separation between enantiomers (Pathirathna et al., 2005). The 5α-reduced steroids also exhibited analgesic effects in rodent models of peripheral pain, and this effect showed strong enantioselectivity. Importantly, T-channel activity is critical for peripheral anti-nociception, but activation of GABAA channels alone can enhance analgesia without altering baseline pain processing. Enantioselectivity for effects on T-channels extended to a steroid analogue that lacks significant effects on GABAARs (Todorovic et al., 1998).
The high degree of enantioselectivity of AlloP and other 3α-hydroxyl, 5α-reduced pregnanes on GABAARs and T-channels stands in distinct contrast to a lack of difference between the effects of enantiomers on lipid bilayers. In early studies, the enantiomers of both AlloP and pregnanolone produced indistinguishable effects on the physical properties of lipid bilayers of different composition (Alakoskela et al., 2007). This important set of results effectively excludes changes in membranes as being critical or relevant for anesthetic effects or effects on GABAARs and T-channels.
5.3. Intracellular accumulation and effects of ent-pregnanes
Subsequent studies with novel enantiomeric pairs of NAS provided additional insights into membrane and intracellular accumulation. In one set of studies, cellular and membrane accumulation and effects on GABAARs were studied using a 7-nitrobenz-2-oxa-1,3-diazole (NBD)-tagged fluorescent analogue of AlloP and its enantiomer. The steroid with the configuration of natural AlloP is a potent and effective modulator of GABAARs, while its unnatural enantiomer is ineffective at these receptors (Chisari et al., 2009). However, these two steroids demonstrated identical fluorescence accumulation in membranes and neurons. An NBD-tagged fluorescent analogue of the anesthetic steroid, alphaxalone, which has lower lipophilicity compared to AlloP, showed faster accumulation and departitioning from cell membranes and intracellular compartments. Using analysis of NAS with different degrees of lipophilicity, other studies demonstrated a relationship between hydrophobicity and potency for effects on GABAARs, indicating that aqueous concentration is not an accurate indicator of NAS binding affinity for the receptors and supporting the hypothesis that membrane accumulation contributes significantly to potency and duration of actions, likely by enhancing access to the receptors (Chisari et al., 2009, 2010; Shu et al., 2004). Importantly, these studies provided strong support for the hypothesis that both lipophilicity and pharmacophore considerations are important in the effects of NAS GABAAR PAMs.
Other studies used a series of NAS analogues with less complex chemical substitutions that allowed use of click chemistry to label steroids bio-orthogonally following administration to track cellular accumulation and effects on GABAARs. A clickable analogue based on AlloP effectively modulated GABAARs and selectively labelled intracellular Golgi in cultured rat hippocampal neurons (Jiang et al., 2016). This distribution of the steroid was mimicked by its enantiomer and did not require cellular energy, although the enantiomer was inactive at GABAARs. Furthermore, the distribution of the GABA-active steroid differed from cholesterol and was not mimicked completely by other non-enantiomeric structural analogues that were inactive at GABAARs (Jiang et al., 2016). These initial studies also provided evidence that accumulation of NAS in neuronal cell bodies can act as either a sink or a source of NAS on somatic GABAARs. The GABAergic clickable NAS was subsequently used in photoaffinity labeling studies to determine amino acid residues on GABAAR subunits that interact with NAS and other cellular targets of NAS (see below) (Chen et al., 2019; Cheng et al., 2019).
5.4. Neuroprotective effects of ent-pregnanes
The studies outlined above indicate that enantiomers of AlloP analogues serve as useful monitors of non-specific effects of NAS (particularly intramembranous and intracellular accumulation), but other work with these steroids indicates that the unnatural AlloP enantiomer has important effects, independent of GABAARs that could have relevance for neurotherapeutics. An intriguing, but incompletely understood property of AlloP is its ability to protect neurons in models of neurodegeneration. These protective effects include a murine model of Niemann-Pick C (NPC) disease that results in developmental neurodegeneration and involves constitutive knockout of the npc gene (npc−/− mice) (Griffin et al., 2004). In both humans and mice, the NPC phenotype results in changes in neuronal lipid storage, defective neurosteroidogenesis, Purkinje cell loss in the cerebellum and premature death. In the mouse model, all knockout animals die by 12–14 weeks of age. Intriguingly, a single injection of AlloP (25 mg/kg sc) at postnatal day 7 (PND 7), during the period of developmental synaptogenesis, extends lifespan by over 40% (with death occurring by about 18 weeks of age) (Griffin et al., 2004; Langmade et al., 2006). This dose of AlloP causes acute sedation lasting about 45 min. The knockout mice also exhibit loss of Purkinje cells and neuroinflammatory changes with microglia invasion and production of proinflammatory cytokines including tumor necrosis factor-α (TNFα) and interleukin 1β (IL1 β). All of these cellular changes are dampened significantly by the single AlloP injection on PND 7. Remarkably, the identical dose of ent-AlloP at PND 7 produced similar neuroprotection, survival and effects on inflammatory changes without causing sedation following acute injection (the latter being a GABAergic effect). Furthermore, both enantiomers activated pregnane X receptors, nuclear receptors involved in regulating genes that help detoxify cells and promote expression of pro-survival factors, suggesting a possible contributing mechanism (Lamba et al., 2004; Frye et al., 2014; Wnuk and Kajta, 2017). AlloP, unlike certain oxysterols, did not activate liver X receptors, a related class of nuclear receptors (Langmade et al., 2006). In cell culture studies, the AlloP enantiomers were equally protective against oxidative stress induced by NPC mutation or knockdown (Zampieri et al., 2009).
ent-AlloP is also neuroprotective in ex vivo and in vivo rodent models of glaucoma. In the ex vivo model, dissected rat eye cups are acutely incubated under varying degrees of pressure for 24 h. At 10 mm Hg of ambient pressure (akin to normal conditions in the eye) retinas have normal histological appearance; high pressure (75 mm Hg) results in degenerative changes in retinal ganglion cells (RGCs) and swelling of their axons. Treatment with 1 μM AlloP during the 24 h incubation results in complete preservation of retinal histology (Ishikawa et al.,2021). Remarkably, 1 μM ent-AlloP produces identical protection (Ishikawa et al., 2022). Effects of natural AlloP involve both activation of GABAARs and stimulation of the cellular process of autophagy. GABAARs do not contribute to the effects of ent-AlloP, yet ent-AlloP effectively induces autophagic flux leading to retinoprotection (Ishikawa et al., 2022). These effects of AlloP enantiomers extend to an in vivo glaucoma model. The in vivo model involves a 21 day experiment that begins with intravitreal injection of microbeads into the anterior eye chamber. Over the first three days after injection intraocular pressure increases from about 10 mm Hg to 30 mm Hg and remains elevated at this level over the remainder of the experiment. The increased intraocular pressure damages ganglion cells and their axons with diminished retinal function as measured by the scotopic threshold response. A single intravitreal injection of either AlloP or ent-AlloP on day 7 of the experiment produces complete histological protection of the retina and preservation of retinal function. Both AlloP and ent-AlloP promote autophagic flux in the in vivo model contributing substantially to neuroprotection with the unnatural enantiomer showing greater effectiveness in autophagy activation (Covey et al., 2022; Ishikawa et al., 2021, 2022).
The protective mechanisms of AlloP enantiomers are incompletely understood. Photoaffinity labeling studies using some of the NAS outlined above have identified several potential targets that could be important. These include voltage dependent anion channels (VDACs) that are labelled at Glu-73, a site that also binds cholesterol (Cheng et al., 2019). VDACs are important contributors to the mitochondrial permeability transition pore that helps to regulate cell death programs. A second site previously identified is on β-tubulin at Cys-354, the same site also labelled by colchicine (Chen et al., 2012a, 2012b). It is unknown whether effects on this key microtubule protein contributes to anti-inflammatory or other effects of AlloP. Presently it is also unknown whether effects on VDAC or β-tubulin are enantioselective.
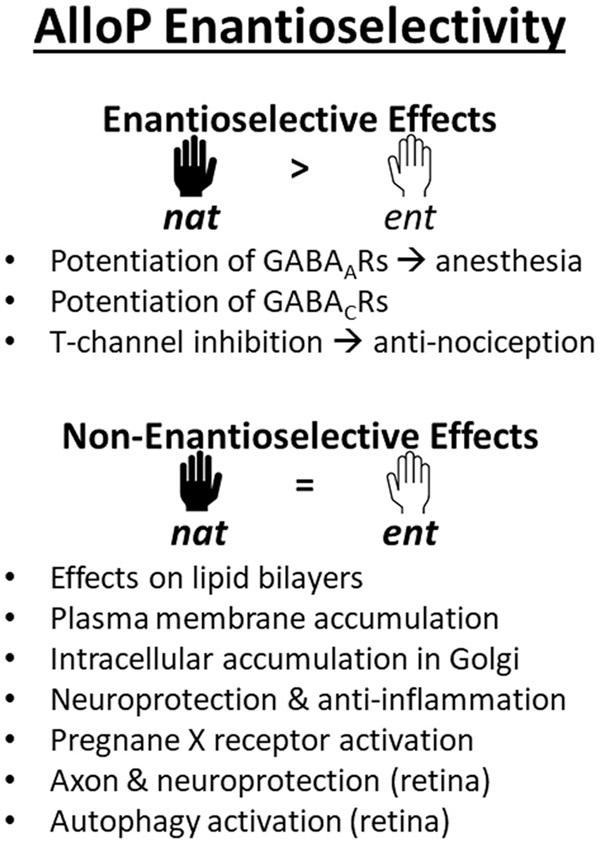
Taken together studies of enantiomeric pairs of pregnane steroids have been highly informative, indicating certain effects that are unequivocally enantioselective (potentiation of GABAARs and sedation, and inhibition of T-channels and peripheral analgesia) and effects that show no enantioselectivity (membranous and intracellular accumulation, and neuroprotection in two models studied to date). A summary of enantioselective effects of AlloP and derivatives is shown in Fig. 3. These findings raise important opportunities in the potential development of enantiomers as therapeutic agents.
Fig. 3.
The figure summarizes various effects where the enantiomers of AlloP have been compared, including effects with enantioselectivity (nat > ent), and effects where the enantiomers are equivalent (nat = ent). See text for details and references.
6. Androstanes
6.1. Effects of androstanes in the nervous system
Regarding the naming of steroids, pregnane and androstane classes are distinguished by the presence of a two-carbon side chain at the C-17 position on the steroid D-ring of the former. While this nomenclature rule is salient for indicating the different hormonal activities of these two classes of steroids, it does not predict differences in their non-hormonal activities. Thus, many analogues of AlloP that lack the pregnane side chain will be named as androstanes even though their actions on ion channels are similar to the pregnanes. For this reason, we discuss steroids and their enantiomers in both classes sequentially because they have qualitatively similar actions on ion-channels, particularly GABAARs, and potentially similar clinical utility. In general, the enantiomers of steroid hormones lack hormonal activities and are not substrates for enzymes involved in hormone biosynthesis. The literature on the enzymology and physiology of ent-steroid hormones was reviewed in detail by Biellmann in 2003.
6.2. Effects of ent-androstanes
Two androstane steroids are of particular interest based on effects of their enantiomers. The natural steroids, androsterone and etiocholanolone differ from AlloP and its derivatives in being weak potentiators of GABAARs. These weaker effects likely result from differences in substitutions at the C-17 position of the steroid D-ring (Katona et al., 2008). In contrast, the unnatural enantiomers of these androstanes were found to be surprisingly potent and effective modulators of GABA receptors (Table 1) (Katona et al., 2008). Single channel recordings and site directed mutagenesis studies indicate that the enantiomers of etiocholanolone may each act at distinct sites on GABAARs (Li et al., 2007).
Other structure-activity studies pursuing features of androstane enantiomers that contribute to effectiveness at GABA receptors made several important observations. These include the presence of a ketone group at position C-16, a C-18 methyl group and an axial methoxyl substitution at the 4α-position (Qian et al., 2014). Additionally, enantiomers with these substitutions were more potent and effective in producing anesthetic effects in tadpoles and mice. In other studies, the enantiomers of androsterone and etiocholanolone were found to be anticonvulsants, consistent with their enhanced GABAergic activity and with more potent and longer durations of actions than the natural steroids in vivo (Zolkowski et al., 2014). These results strongly suggest that certain enantiomeric androstanes could be developed for therapeutic purposes based on GABAergic activity, including possible antidepressant, anxiolytic, anesthetic, anticonvulsant, analgesic, neurogenic and neuroprotective effects (Melcangi et al., 2021; Kelava et al., 2022).
7. Estrogens
7.1. Effects of estrogens in the nervous system
Neuroactive estrogens, particularly 17β-estradiol (17β-E2), represent another important class of neurosteroids that modulate multiple brain functions including behavior, neuronal structure, neural excitability and survival (Woolley et al., 2007; Simpkins et al., 2013; Brann et al., 2022). These steroids can be synthesized locally in brain from cholesterol via pregnenolone in both neurons and glia (Belelli and Lambert, 2005; Fester and Rune, 2021) and act at classical estrogen receptors (ERα and ERβ) that are expressed both intracellularly and on the surface of cells. Classical intracellular ERs play key roles in mediating hormonal actions in the periphery (Farkas et al., 2022). Acutely, estrogens can modify intrinsic neuronal physiology and synaptic function of both excitatory (glutamatergic) and inhibitory (GABAergic) transmission by presynaptic and postsynaptic effects acting on specific receptors expressed on the surface of neurons and other cells (Huang and Woolley, 2012; Oberlander and Woolley, 2016). Furthermore, 17β-E2 and derivatives have powerful neuroprotective effects in several preclinical models in vitro and in vivo, including models of oxidative stress, cerebral trauma, ischemia and neurodegenerative illnesses, among others (Brann et al., 2022; Farkas et al., 2022; Engler-Chiurazzi et al., 2017; Saeed et al., 2021).
There is considerable information about structure-activity relationships of 17β-E2 and derivatives at classical estrogen receptors, likely because of the importance of these steroids in reproductive biology, behavior and pharmacology. Less is known about structure-activity requirements for estrogens on neuronal function and excitability. In contrast, detailed structure-activity studies have explored how 17β-E2 produces neuroprotection. These studies have shown that estrogenmediated neuroprotection in preclinical models can occur independently of effects on classical ERs. The steroid A-ring, particularly its phenolic structure, is a minimal requirement for neuroprotection (Engler-Chiurazzi et al., 2017); methylation of the phenolic A-ring hydroxyl group eliminates protective effects. However, neuroprotection is enhanced by substitution of non-polar groups at the C2 and C4 carbons of the A-ring (Qian et al., 2016). Mechanistically, 17β-E2 neuroprotection involves synergistic modulation of the antioxidant effects of glutathione via generation of NADPH from the hexose monophosphate shunt, resulting in increased levels of reduced forms of glutathione (Dykens et al., 2004). Importantly, these detailed structure-activity studies provided strong support for the hypothesis that neuroprotective effects can be separated from classical feminizing actions of estrogens.
7.2. Effects of ent-estrogens
The enantiomer of 17β-E2 has been studied in a variety of models since the 1960 s and 1970 s (Terenius, 1968; Chernayaev et al., 1975; Payne et al., 1979). This enantiomer interacts weakly with classical ERs and lacks estrogen-like effects on reproductive tissues in animals. Only limited information is available about brain effects of ent-17β-E2. 17β-E2 potentiates certain neuronal nicotinic receptors, and this effect is enantioselective (Paradiso et al., 2001). These same nicotinic receptors are enantioselectively inhibited by a 3α-hydroxyl, 5α-reduced androstane with about three-fold separation between enantiomers (Paradiso et al., 2000).
Importantly, ent-17β-E2 shows highly effective neuroprotection in models of neurodegeneration including toxicity produced by hydrogen peroxide and oxidative stress in a hippocampal cell line model and in a neuroblastoma cell line in vitro. The E2 enantiomer is also effective in an in vivo rodent model of focal cerebral ischemia involving occlusion of the middle cerebral artery (Green et al., 2001). In both the in vitro and in vivo models, the enantiomer of 17β-E2 was equally effective and as potent as natural 17β-E2, but exhibited no stimulation of uterine growth or effects on vaginal opening in juvenile female rats, indicating a lack of effect on classical estrogen receptors. Furthermore, in cultured rat cortical neurons, both 17β-E2 and its enantiomer protect against glutamate toxicity via a non-ER-dependent mechanism by preserving serine phosphatase activity and dampening pathological kinase activation and resultant oxidative stress. Neither enantiomer was effective against toxicity in the presence of specific phosphatase inhibitors (YI et al., 2008, 2009). Taken together, these findings indicate that ent-17β-E2 has therapeutic potential as a neuroprotectant without the feminizing effects of the natural steroid. At present there is no information about ent-17β-E2 on other brain effects of estrogens including changes in excitability.
8. Sulfated steroids
8.1. Effects of sulfated neurosteroids in the nervous system
Most neurosteroids are unconjugated, but are subject to a variety of enzymatic modifications that affect their activities and targets. Certain neurosteroids can be sulfated by endogenous sulfotransferases and this generates molecules with interesting effects in brain. Sulfated steroids, most notably pregnenolone sulfate (PREGS) and dehydroepiandrosterone sulfate (DHEAS), modulate certain ion channels to alter neuronal excitability and synaptic transmission, and different sulfated steroids can be positive or negative allosteric modulators. Known targets of sulfated steroids include ligand gated ion channels such as GABAARs, glycine receptors, N-methyl-D-aspartate receptors (NMDARs), kainic acid receptors and AMPA receptors, mediators of the majority of fast (phasic) and tonic excitation and inhibition in the nervous system (Kudova, 2021; Vitku et al., 2022). Sulfated neurosteroids can also regulate the activity of certain GPCRs, transient receptor potential (TRP) channels and sigma 1 receptors (S1Rs). These steroids, in turn modulate learning and memory, neuronal survival, neurite outgrowth and neurogenesis. Sulfation increases water solubility of these agents and alters their half-lives, making sulfated steroids potential targets for drug development, although delivery of these charged molecules across the blood-brain barrier is a significant challenge.
Presently, most information about sulfated neurosteroids involves the effects of PREGS (and to a lesser extent DHEAS). PREGS and DHEAS are sometimes referred to as “excitatory neuroactive steroids” based on their effects on ion channels (Vitku et al., 2022). PREGS is an effective PAM at NMDARs, but is a negative allosteric modulator (NAM) of GABAARs, giving it unique actions by which it can modulate the balance of brain excitation and inhibition. Notably, effects on NMDA receptors are generally less potent than effects on GABAARs. Certain subtypes of NMDARs are more sensitive to potentiation by PREGS, including NMDARs that express GluN2A and GluN2B subunits, with lower potency and effectiveness at receptors with GluN2C and GluN2D subunits (Horak et al., 2006). PREGS can also inhibit NMDARs under certain conditions, complicating interpretation of effects (Horak et al., 2006). A 5β-reduced analogue of PREGS (pregnanolone sulfate, PAS) is a negative modulator of NMDARs (Kapras et al., 2018). At GABAARs, PREGS is a potent, activation-dependent, non-competitive inhibitor (Park-Chung et al., 1999; Seljeset et al., 2018) and appears to act, at least in part, by enhancing receptor desensitization (Shen et al., 2000; Eisenman et al., 2003). PREGS also shows little GABAAR subtype selectivity except for rho-1 receptors that are less sensitive to inhibition (Seljeset et al., 2018).
8.2. Effects of enantiomers of sulfated neurosteroids
Enantiomers of sulfated steroids were synthesized in the late 1990′s (Nilsson et al., 1998) and most studies have examined the effects of ent-PREGS, an agent that has clear but complex effects on brain function, with some actions showing high degrees of enantioselectivity of the natural steroid, other effects showing no difference between enantiomers and some effects showing reverse enantioselectivity. Early studies found that the ability of PREGS to inhibit GABAARS (Nilsson et al., 1998) and to potentiate NMDARs (Vallee et al., 2001) showed no enantioselectivity. Additionally, PREGS enantiomers show no differences in effects on membrane capacitance, as expected for these lipophilic agents with the same physicochemical properties (Mennerick et al., 2008). In contrast, effects of DHEAS on GABAARs surprisingly showed about 8-fold separation with higher potency of the natural enantiomer (Nilsson et al., 1998). The 5β-reduced sulfated steroid, PAS, is a negative modulator of GABAARs and shows no enantioselectivity (Nilsson et al., 1998), although the unnatural enantiomer was about 2.6-fold less potent than PAS for inhibitory effects on NMDARs (Kapras et al., 2018). Effects of PREGS on activation of TRPM3 (Transient Receptor Potential Melastatin 3 channels) showed enantioselectivity favoring the natural enantiomer, while inhibition of PAORAC (proton-activated outwardly rectifying anion channels) showed no enantioselectivity, akin to GABA-gated chloride channels described above (Drews et al., 2014). Effects of sulfate enantiomers on other receptors and channels have not been reported to date.
PREGS enantiomers have been examined on synaptic transmission and in models of learning and memory and neurodegeneration. At hippocampal glutamatergic synapses, PREGS augments the frequency, but not the amplitude of AMPAR-mediated miniature excitatory postsynaptic currents (mEPSCs) in hippocampal cultures, and also decreases paired-pulse facilitation of evoked EPSCs, indicating a presynaptic increase in release of glutamate at these synapses. Enhancement of mEPSCs was mimicked by DHEAS but not by ent-PREGS, and was blocked by inhibitors of sigma 1 receptors (S1Rs), suggesting that PREGS acts on these receptors in an enantioselective fashion (Meyer et al., 2002).
Enhancing effects of PREGS on NMDARs and glutamate transmission are consistent with other studies that have examined effects of PREGS on memory, and the unnatural PREGS enantiomer has also been examined in several studies of memory and learning. Akwa et al. (2001) examined effects of the enantiomers in a spatial memory task involving two-trial recognition in a novel environment Y-maze test. In this study, both enantiomers enhanced memory performance following post-acquisition intracerebroventricular (icv) administration, but surprisingly, ent-PREGS was about 10-fold more potent than PREGS. Furthermore, pro-memory effects of PREGS, but not ent-PREGS were blocked by the NMDAR antagonist, 2-amino-5-phosphonovalerate (APV), indicating that effects of the enantiomer were independent of NMDARs. Consistent with this, a subsequent study using the two-trial Y-maze discrimination task found that memory enhancement by PREGS, but again not ent-PREGS, was eliminated in mice with knockout of the obligatory GluN1 NMDAR subunit (Petit et al., 2011). These results indicate that there is reverse enantioselectivity for memory enhancement by PREGS in at least some tasks and that ent-PREGS can act by a mechanism independent of NMDARs.
The PREGS enantiomers have also been examined against amnesic effects of scopolamine (an anti-muscarinic drug) in a passive avoidance memory task. Here, both enantiomers blocked the effects of scopolamine, but PREGS was about 10-fold more potent than ent-PREGS following icv administration 30 min prior to training (Vallee et al., 2001). Mechanisms underlying the effects of the enantiomers in these scopolamine studies are unknown, but do not appear to involve well-known effects on either NMDA or GABA receptors. Also, in contrast to the studies outlined above, these results indicate clear enantioselectivity favoring the natural neurosteroid.
In a cell culture model of beta-amyloid (Aβ25–35) neurotoxicity in B104 cells, both enantiomers of PREGS and DHEAS diminished cell loss and stimulated neurite outgrowth (El Bitar et al., 2014). Natural PREGS was more potent than its enantiomer but DHEAS showed no enantioselectivity. The enantiomers were also studied in an in vivo model using icv administration of Aβ25–35 following pretreatment with the steroids. In this model, both ent-PREGS and ent-DHEAS, in contrast to the natural neurosteroids, effectively diminished Aβ25–35-induced memory impairment in spontaneous alternation and passive avoidance tasks when administered 6 h prior to Aβ25–35. The enantiomers also decreased lipid peroxidation, a marker of cell stress and injury (El Bitar et al., 2014). Mechanisms underlying these protective effects are unknown, but the results support the potential use of these enantiomeric steroids as neuroprotectants.
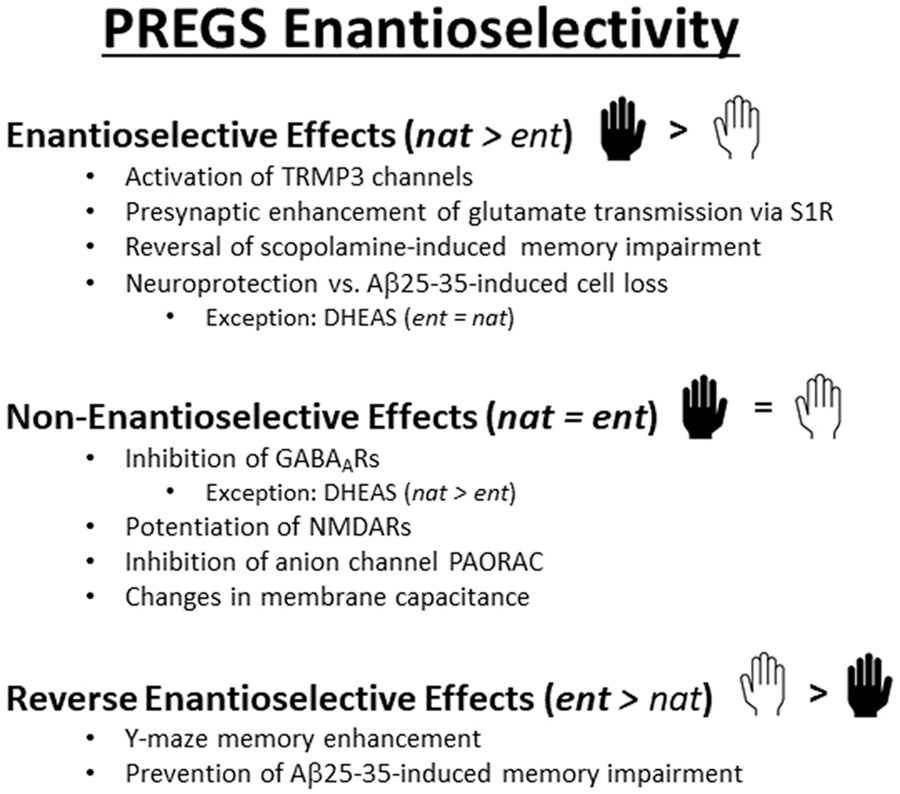
Taken together, these results indicate that ent-PREGS (and likely ent-DHEAS) have intriguing effects on brain function including memory enhancing actions and neuroprotection in several model systems. A summary of the effects of the sulfated neurosteroid enantiomers is shown in Fig. 4.
Fig. 4.
Various effects of PREGS and its enantioselectivity are depicted and highlight effects with enantioselectivity (nat > ent), no enantioselectivity (nat = ent) and reverse enantioselectivity (ent > nat). The figure also shows complex effects of the DHEAS enantiomers. See text for details and references.
9. Summary & conclusions
While detailed studies of unnatural neurosteroid enantiomers are a work in progress, it is clear that these agents have potentially important effects on brain function that could be harnessed for treatment of a variety of neuropsychiatric illnesses. Several of these agents have potent neuroprotective actions that rival and, in some cases, exceed the effects of the natural neurosteroids. Some enantiomers also appear to have unique mechanisms compared to natural steroids (e.g. PREGS). As highlighted in this review, specific examples with neuroprotective properties include the enantiomers of progesterone, AlloP, 17β-estradiol and possibly PREGS. The enantiomers of the androgens, androsterone and etiocholanolone, are potent positive allosteric modulators of GABAARs and could provide unique alternatives for treatment of disorders in which enhanced inhibition is desired, including epilepsy, sleep, anxiety and pain. Proof of concept studies in rodents support anticonvulsant properties of the androstane enantiomers (Zolkowski et al., 2014), but it is presently unknown whether they are neuroprotective.
Certain enantiomeric NAS have potentially important beneficial effects on learning and memory including prevention and/or reversal of memory defects in pathological conditions. Some of these effects may involve neuroprotective mechanisms and anti-cellular stress effects, including dampening of neuroinflammation and antioxidant properties. Given the importance of cognitive defects in driving disability and dysfunction in numerous neuropsychiatric illnesses, the enantiomeric steroids could play key roles in preventing or reversing these devastating outcomes.
Why enantiomers? Beyond potentially important effects on brain function outlined above, the enantiomers have certain properties that could enhance their attractiveness as therapeutics. These include a lack of agonist activity mediated by steroid nuclear hormone receptors as reported for the enantiomers of progesterone and 17β-estradiol. Additionally, the lack of effects of certain enantiomers such as ent-AlloP on GABA channels would be expected to avoid sedation and potentially adverse effects on cognition that can come with broadly enhanced GABAergic function. The enantiomers will also likely have major differences in their in vivo metabolism that could prolong half-lives as drugs and prolong key activities, as has been observed in studies of the anticonvulsant effects of certain ent-androgens (Zolkowski et al., 2014). Furthermore novel mechanisms of enantiomers may open new directions in therapeutics. This latter concept is not unprecedented and current efforts to understand enantiomeric metabolites of the rapid antidepressant, ketamine, already appear to offer potentially new approaches to treatment (Highland et al., 2021; Bonaventura et al., 2022).
Why not enantiomers? Beyond the fact that only limited information is presently available about the effects of unnatural neurosteroid enantiomers, there are several other considerations that could curtail their development as therapeutics. Enantiomeric steroids are generated by total synthesis and are not accessible at scale from naturally occurring steroids. Newer methods for their synthesis that have the potential to lower the cost for their commercial production have been recently reported (Kim et al., 2018). As noted above, ent-steroids are likely metabolized differently from natural steroids, but little is presently known about this important topic. Differences in the glucuronidation of 17β-estradiol, androsterone and etiocholanolone by human UDP-glucuronosyltransferases have been reported (Sneitz et al., 2011). Whether the enantiomers have additional off-target effects on metabolic or other endogenous pathways remains to be determined. The enantiomers will also have significant effects on cell membranes, but these effects are unlikely to differ from the natural steroids (Alakoskela et al., 2007; Mennerick et al., 2009), and studies to date indicate that enantiomers accumulate and dissipate similarly from cell membranes and intracellular compartments (Chisari et al., 2009, 2010; Jiang et al., 2016). To date there is no information about enantiomeric NAS in humans. Thus it is not clear what side effects will be encountered and how those side effects differ from natural neurosteroids. Future studies are required to determine the translational potential of these compounds for the treatment of disorders where NAS have been suggested to have benefit, such as anxiety, depression, epilepsy, and others (Belelli et al., 2020; Goodchild et al., 2020; Johansson et al., 2016; Reddy and Estes, 2016; Zorumski et al., 2019).
In summary, enantiomeric neuroactive steroids have proven to be useful scientific tools in a wide range of studies of neuronal function, and have stepped out of the shadows of their natural partners to demonstrate major positive effects on their own. These findings raise intriguing possibilities about how the positive effects of these agents may be harnessed for therapeutic uses in neuropsychiatry.
Acknowledgements
Work in the authors laboratories is supported by National Institutes of Health grants MH122379 (DFC, ASE, JLM, SJM and CFZ), MH101874 (SJM, CFZ), MH123748 (SJM), MH110550 (DFC), R01AA026256 (JLM), R01NS105628 (JLM), R01NS102937 (JLM), R01MH128235 (JLM), GM108799 (ASE), the Taylor Family Institute for Innovative Psychiatric Research and the Bantly Foundation.
Nomenclature
- 17β-E2
17β-estradiol
- 17PA
3α,5α-17-phenylandrost-16-en-3-ol
- Aβ25–35
beta-amyloid 25–35
- AlloP
allopregnanolone
- CNS
central nervous system
- DHEAS
dehydroepiandrosterone sulfate
- ent
enantiomeric configuration steroid
- EPSC
excitatory postsynaptic current
- ERα and ERβ
estrogen receptor α and β
- GABA
gamma aminobutyric acid
- GABAAR
gamma aminobutyric acid type A receptor
- GPCR
G-protein coupled receptor
- icv
intracerebroventricular
- IL1β
interleukin 1β
- MDD
major depressive disorder
- mEPSC
miniature excitatory postsynaptic current
- mPR
membrane progesterone receptors
- NAM
negative allosteric modulator
- NAS
neuroactive steroids
- na
natural configuration steroid
- NBD
7-nitrobenz-2-oxa-1,3-diazole
- NMDAR
N-methyl-D-aspartate receptor
- NPC
Niemann-Pick C disease
- PAM
positive allosteric modulator
- PAORAC
proton-activated outwardly rectifying anion channels
- PAS
pregnanolone sulfate
- PGRMC1
PROG receptor membrane component 1
- PND 7
postnatal day 7
- PPD
postpartum depression
- PREGS
pregnenolone sulfate
- PROG
progesterone
- PRs
progesterone receptors
- RGC
retinal ganglion cells
- ROS
reactive oxygen species
- S1R
sigma 1 receptor
- TBI
traumatic brain injury
- TBPS
tert-butylbicyclophosphorothionate
- TNFα
tumor necrosis factor-α
- TRPM3
Transient Receptor Potential Melastatin 3 channels
- VDAC
voltage dependent anion channel.
Footnotes
Conflict of interest statement
CFZ and JLM serve on the Scientific Advisory Board of Sage Therapeutics; DFC and CFZ have equity in Sage Therapeutics. The other authors have no conflicts to disclose.
Data Availability
No data was used for the research described in the article.
References
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A, 2006. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci 103, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE, 2001. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc. Natl. Acad. Sci 98, 14033–14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakoskela JM, Covey DF, Kinnunen PK, 2007. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim. Biophys. Acta 1768, 131–145. [DOI] [PubMed] [Google Scholar]
- Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, Kazdoba TM, Modgil A, Davies PA, Moss SJ, Salituro FG, Hoffmann E, Hammond RS, Robichaud AJ, Quirk MC, Doherty JJ, 2020. Preclinical characterization of zuranolone (SGE-217), a selective neuroactive steroid GABA-A receptor positive allosteric modulator. Neuropharmacol 181, 108333. 10.1016/j.neuropharm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ, Kumar AS, Boswell CA, Gupta MK, Bruce K, Rath NP, Covey DF, 2003. The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch. Biochem. Biophys 409, 134–144. [DOI] [PubMed] [Google Scholar]
- Bassani TB, Bartolomeo CS, Oliveira RB, Ureshino RP, 2022. Progestogen-mediated neuroprotection in central nervous system disorders. Neuroendocrinol. 10.1159/000525677. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, 1981. Steroid hormones in the brain: several mechanisms. In: Fuxe K, Gustafsson J-A, Wetterberg L (Eds.), in Steroid Hormone Regulation of the Brain. Pergamon, Oxford, pp. 3–14. [Google Scholar]
- Belelli D, Lambert JJ, 2005. Neurosteroids: endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci 6, 565–575. [DOI] [PubMed] [Google Scholar]
- Belelli D, Hogenkamp D, Gee KW, Lambert JJ, 2020. Realising the therapeutic potential of neuroactive steroid modulators of the GABAa receptor. Neurobiol. Stress 12, 100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biellmann J-F, 2003. Enantiomeric steroids: synthesis, physical, and biological properties. Chem. Rev 103, 2019–2033. [DOI] [PubMed] [Google Scholar]
- Bjorkgren I, Mendoza S, Chung DH, Haoui M, Petersen NT, Lishko PV, 2022. The epithelial potassium channel Kir7.1 is stimulated by progesterone. J. Gen. Physiol 153, 3202112924. https://doi.org/10/1085/jgp.202112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S, 2004. Brain cholesterol: long secret life behind a barrier. Arter. Thromb. Vasc. Biol 24, 806–815. [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Gomez JL, Carlton ML, Lam S, Sanchez-Soto M, Morris PJ, Moaddel R, Kang HJ, Zanos P, Gould TD, Thomas CJ, Sibley DR, Zarate CA, Michaelides M, 2022. Target deconvolution studies of (2R,6R)-hydroxynorketamine: an elusive search. Mol. Psychiatry, 10:1038/s41380-022-01673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Lu Y, Wang J, Zhang Q, Thakkar R, Sareddy GR, Pratap UP, Tekmal RR, Vadlamudi RK, 2022. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev 132, 793–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T, Tang M, Jiang P, Zhang B, Wu X, Chen Q, Zeng C, Li N, Zhang S, Cai H, 2021. A potential mechanism underlying the therapeutic effects of progesterone and allopregnanolone on ketamine-induced cognitive defects. Front. Pharmacol. 12, 612083 10.3389/fphar.2021.612083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Bracamontes JR, Budelier MM, Germann AL, Shin DJ, Kathiresan K, Qian MX, Manion B, Cheng WWL, Reichert DE, Akk G, Covey DF, Evers AS, 2019. Multiple functional neurosteroid binding sites on GABAA receptors. PLoS Biol. 17, e3000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Chen LH, Akentieva N, Lichti CF, Darbandi R, Hastings R, Covey DF, Reichert DE, Townsend RR, Evers AS, 2012. A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis 33, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS, 2012T. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABA(A) receptor. Mol. Pharm 82, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WWL, Budelier MM, Sugasawa Y, Bergdoll L, Queralt-Martín M, Rosencrans W, Rostovtseva TK, Chen ZW, Abramson J, Krishnan K, Covey DF, Whitelegge JP, Evers AS, 2019. Multiple neurosteroid and cholesterol binding sites in voltage-dependent anion channel-1 determined by photo-affinity labeling. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1864, 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernayaev GA, Barkova TI, Egorova VV, Sorokina IB, Ananchenko SN, Mataradze GD, Sokolova NA, Rozen VB, 1975. A series of optical structural and isomeric analogs of estradiol: a comparative study of the biological activity and affinity to cytosol receptor of rabbit uterus. J. Steroid Biochem 6, 1483–1488. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF, 2010. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 33, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S, 2009C. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low affinity interaction. J. Neurophysiol 102, 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu E-E, 1981. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci 78, 4704–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, 2009. ent-steroids: novel tools for studies of signaling pathways. Steroids 74, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Evers AS, Mennerick S, Zorumski CF, Purdy RH, 2001. Recent developments in structure-activity relationships for steroid modulators of GABA(A) receptors. Brain Res. Rev 37, 91–97. [DOI] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS, 2000. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acidA receptor modulation and anesthesia. J. Pharmacol. Exp. Ther 293, 1009–1016. [PubMed] [Google Scholar]
- Covey D, Zorumski C, Izumi Y, Ishikawa M, 2022. US 11,497,754, Neurosteroids and enantiomers thereof for the prevention and treatment of neurodegenerative conditions. November 15, 2022.
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, Kanes SJ, Lasser R, 2021. Effect of zuranolone vs placebo in postpartum depression – a randomized clinical trial. JAMA Psychiatry 78, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews A, Mohr F, Rizun O, Wagner TFJ, Dembla S, Rudolph S, Lambert S, Konrad M, Philipp SE, Behrendt M, Marchais-Oberwinkler S, Covey DF, Oberwinkler J, 2014. Structural requirements of steroidal agonists of transient receptor potential melastatin 3 (TRPM3) cation channels. Br. J. Pharmacol. 171, 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA, Carroll AK, Wiley S, Covey DF, Cai ZY, Zhao I, Wen R, 2004. Photoreceptor preservation in the S334ter model of retinitis pigmentosa by a novel estradiol analog. Biochem. Pharmacol 68, 1971–1984. [DOI] [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S, 2003. Activation dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J. Physiol. (Lond. ) 550, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bitar F, Meunier J, Villard V, Almeras M, Krishnan K, Covey DF, Maurice T, Akwa Y, 2014. Neuroprotection by the synthetic neurosteroid enantiomers ent-PREGS and ent-DHEAS against A-25-35 peptide-induced toxicity in vitro and in vivo in mice. Psychopharmacol 231, 3293–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Covey DF, Simpkins JW, 2017. A novel mechanism of nonfeminizing estrogens in neuroprotection. Exp. Gerontol 94, 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers AS, Covey DF, Zorumski CF, 2000. Enantioselectivity of steroid anesthetic action. Prog. Anesth. Mech 6, 372–381. [Google Scholar]
- Farkas S, Szabo A, Hegyi AE, Torok B, Fazekas CL, Ernszt D, Kovacs T, Zelena D, 2022. Estradiol and estrogen-like alternative therapies in use: the importance of the selective and non-classical actions. Biomedicines 10, 861. 10.3390/biomedicines10040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Rune GM, 2021. Sex neurosteroids: hormones made by the brain for the brain. Neurosci. Lett 753, 135849. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA, 2014. Involvement of pregnane xenobiotic receptor in mating-induced allopregnanolone formation in the midbrain and hippocampus and brain-derived neurotrophic factor in the hippocampus. Psychopharmacol 231, 3375–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani R, Hoffman SW, Stein DG, 2001. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor. Neurol. Neurosci 8, 161–166. [PubMed] [Google Scholar]
- Ghandiri T, Vakilzadeh G, Hajali V, Khodagholi F, 2019. Progesterone modulates post-traumatic epileptogenesis through regulation of BDNF-TrkB signaling and cell survival-related pathways in the rat hippocampus. Neurosci. Lett 70, 13484. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ghanem CA, Asbelaoui N, Schumacher M, Hussain R, 2020. Roles of progesterone, testosterone and their nuclear receptors in central nervous system myelination and remyelination. Int. J. Mol. Sci 21, 3163. 10.3390/ijms21093163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SL, Coronel MF, Raggio MC, Labombarda F, 2020. Progesterone receptor-mediated actions and the treatment of central nervous system disorders: an up-date of the known and the challenge of the unknown. Steroids 153, 108525. 10.1016/j.steroids.2019/108525, 2020. [DOI] [PubMed] [Google Scholar]
- Goodchild CS, Serrao JM, Sear JW, Anderson BJ, 2020. Pharmacokinetic and pharmacodynamic analysis of alphaxalone administered as a bolus intravenous injection of Phaxan in a Phase 1 randomized trial. Anesth. Analg 130, 704–714. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang S-H, Nilsson KR, Kumar AS, Covey DF, Simpkins JW, 2001. The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinol 142, 400–406. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon S, 2004. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med 7, 704–711. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS, 2002. Progesterone withdrawal increases the β4 subunit of the GABAA receptor in male rats in association with anxiety and altered pharmacology – a comparison with female rats. Neuropharmacol 43, 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, Li H, Lasser R, Zorumski CF, Rubinow DR, Paul SM, Jonas J, Doherty JJ, Kanes SJ, 2019. Trial of SGE-217 in patients with major depressive disorder. New Engl. J. Med 381, 903–911. [DOI] [PubMed] [Google Scholar]
- Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, Moaddel R, Thomas CJ, Zarate CA, Pereira EFR, Gould TD, 2021. Hydroxynorketamines: pharmacology and potential therapeutic applications. Pharmacol. Rev 73, 763–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Chodounska H, Vyklicky L, 2006. Subtype-dependence of N-methyl-D-aspartate receptor modulation by pregnenolone sulfate. Neuroscience 137, 93–102. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG, 2006. Endogenous neurosteroids regulate GABAA receptors via two discrete transmembrane sites. Nature 444, 486–489. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wittmer LL, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF, 1997. Neurosteroid analogues. Part 5. Enantiomers of neuroactive steroids and benz[e] indenes: total synthesis, electrophysiological effects on GABAa receptor function and anesthetic actions in tadpoles. J. Chem. Soc. Perkin Trans 1, 3665–3671. [Google Scholar]
- Huang GZ, Woolley CS, 2012. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Nakazawa T, Kunikata H, Sato K, Yoshitomi Y, Krishnan K, Covey DF, Zorumski CF, Izumi Y, 2022. The enantiomer of allopregnanolone prevents pressure-mediated retinal degeneration via autophagy. Front. Pharmacol 13, 855779 10.3389/fphar.2022.855779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Takaseki S, Yoshitomi T, Covey DF, Zorumski CF, Izumi Y, 2021T. The neurosteroid allopregnanolone protects retinal neurons by effects on autophagy and GABRs/GABAA receptors in rat glaucoma models. Autophagy 17, 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska I, Covey DF, 2022. Chiral building blocks for total steroid synthesis and the use of steroids as chiral building blocks in organic synthesis. In: Wojaczynska E, Wojaczynski J (Eds.), Chiral Building Blocks in Asymmetric Synthesis. WILEY-VCH Gmbh, Weinheim, Germany, pp. 367–388. [Google Scholar]
- Jiang X, Shu H-J, Krishnan K, Qian M, Taylor AA, Covey DF, Zorumski CF, Mennerick S, 2016. A clickable neurosteroid photolabel reveals selective Golgi compartmentalization with preferential impact on proximal inhibition. In: Neuropharmacol, 108, pp. 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Stromberg J, Ragagnin G, Doverskog M, Backstrom T, 2016. GABAA receptor modulating steroid antagonists (GAMSA) are functional in vivo. J. Steroid Biochem. Mol. Biol 160, 98–105. [DOI] [PubMed] [Google Scholar]
- Kacher R, Mounier C, Caboche J, Betuing S, 2022. Altered cholesterol homeostasis in Huntington’s disease. Front. Aging Neurosci 14, 797220 10.3389/fnagi.2022.797220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapras V, Vyklicky V, Budesinsky M, Cisarova I, Vyklicky L, Chodounska H, Jahn U, 2018. Total synthesis of ent-pregnanolone sulfate and it biological investigation at the NMDA receptor. Org. Lett 20, 946–949. [DOI] [PubMed] [Google Scholar]
- Kapur J, Joshi S, 2021. Progesterone modulates neuronal excitability bidirectionally. Neurosci. Lett 744, 135619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF, 2008. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABA-A receptors by ent-androgens. Eur. J. Med. Chem 43, 107–113. [DOI] [PubMed] [Google Scholar]
- Kelava I, Chiaradia I, Pellegrini L, Kalinka AT, Lancaster MA, 2022. Androgens increase excitatory neurogenic potential in human brain organoids. Nature 602, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HN, Lee S-J, Koh J-Y, 2012. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem. Int 60, 125–133. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim T-Y, Cho K-S, Kim HN, Koh J-Y, 2013. Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis 59, 80–85. [DOI] [PubMed] [Google Scholar]
- Kim WS, Du K, Eastman A, Hughes RP, Micalizio GC, 2018. Synthetic nat- or ent-steroids in as few as five chemical steps from epichlorohydrin. Nat. Chem. 10, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Manion BD, Taylor A, Bracamontes J, Steinbach JH, Reichert DE, Evers AS, Zorumski CF, Mennerick S, Covey DF, 2012. Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiation site on β-aminobutyric acid type A receptors. J. Med. Chem 55, 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudova E, 2021. Rapid effects of neurosteroids on neuronal plasticity and their physiological and pathological implications. Neurosci. Lett 750, 135771. [DOI] [PubMed] [Google Scholar]
- Lamb Y, 2022. Ganaxolone: first approval. Drugs 82, 933–940. [DOI] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG, 2004. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol 199, 251–265. [DOI] [PubMed] [Google Scholar]
- Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS, 2006. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci 103, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty D, Thomas P, Field M, Andersen OJ, Gold MG, Biggin PC, Gielen M, Smart TG, 2017. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol 24, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G, 2007. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat alpha1 beta2 gamma2L GABA-A receptor. Mol. Pharmacol 71, 1582–1590. [DOI] [PubMed] [Google Scholar]
- Li W, Covey DF, Alakoskela J-M, Kinnunen PKJ, Steinbach JH, 2006. Enantiomers of neuroactive steroids support a specific interaction with the GABA-C receptor as the mechanism of steroid action. Mol. Pharmacol 69, 1779–1782. [DOI] [PubMed] [Google Scholar]
- Liang JJ, Rasmussen AM, 2018. Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic. Stress 2, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-Y, Sun H, Li Q-Y, Lu P-S, 2016. Progesterone for traumatic brain injury: a meta-analysis review of randomized controlled trials. World Neurosurg. 90, 199–210. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM, 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Martinez Botella GM, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco M-J, Belfort GM, Dai J, Loya CM, Ackley MA, Althaus AL, Grossman SJ, Hoffmann E, Doherty JJ, Robichaud AJ, 2017. Neuroactive steroids. 2. 3β-hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (β-aminobutyric acid)A receptor. J. Med. Chem 60, 7810–7819. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Cioffi L, Diviccaro S, Traish AM, 2021. Synthesis and actions of 5β-reduced metabolites of testosterone in the nervous system. Androg.: Clin. Res. Ther 21. 10.1089/andro.2021.0010. [DOI] [Google Scholar]
- Meltzer-Brody S, Kanes SJ, 2020. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurbiol. Stress 200212, 2020. 10.1016/j.ynstr.2020.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF, 2004. Selective antagonism of 5alpha-reduced neurosteroid effects at GABA(A) receptors. Mol. Pharmacol 65, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Lamberta M, Shu H-J, Hogins J, Wang CC, Covey DF, Eisenman LN, Zorumski CF, 2008. Effects on membrane capacitance of steroids with antagonist properties at GABA-A receptors. Biophys. J 95, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela C, 2002. Neurosteroids enhance spontaneous release in hippocampal neurons. Possible role of metabotropic sigma 1-like receptors. J. Biol. Chem 277, 28725–28732. [DOI] [PubMed] [Google Scholar]
- Miller PS, Scott S, Masiulis S, De Colibus L, Pardon E, Steyaert J, Aricescu AR, 2017. Structural basis for GABAA receptor potentiation by neurosteroids. Nat. Struct. Mol. Biol 24, 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D, 2008. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem. Int 52, 588–595. [DOI] [PubMed] [Google Scholar]
- Nilsson KR, Zorumski CF, Covey DF, 1998. Neurosteroid analogues. 6. The synthesis and GABAa receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate and (3β,5β)-3- hydroxypregnane-20-one sulfate. J. Med Chem 41, 2604–2613. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2016. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci 36, 2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH, 2000. Steroid inhibition of rat neuronal nicotinic β4β2 receptors expressed in HEK 293 cells. Mol. Pharmacol 58, 341–351. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH, 2001. The C terminus of the human β4β2 nicotinic receptor forms a binding site required for potentiation by an estrogenic steroid. J., Neurosci 21, 6561–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakala ML, Zhang Y, Modgil A, Chadchankar J, Vien TN, Ackley MA, Doherty JJ, Davies PA, Moss SJ, 2019. Metabotropic, but not allosteric, effects of neurosteroids on GABAergic inhibition depend on the phosphorylation of GABAA receptors. J. Biol. Chem 294, 12220–12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH, 1999. Sulfated and unsulfated steroids modulate β-aminobutyric acidA receptor function through distinct sites. Brain Res. 830, 72–87. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V, 2005. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent analgesic effects of 5alpha-reduced neuroactive steroids. Pain 114, 429–443. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH, 1992. Neuroactive steroids. FASEB J. 6, 2311–2322. [PubMed] [Google Scholar]
- Payne DW, Katzenellenbogen JA, 1979. Binding specificity of rat alpha-fetoprotein for a series of estrogen derivatives: studies using equilibrium and nonequilibrium binding techniques. Endocrinol 105, 745–753. [DOI] [PubMed] [Google Scholar]
- Petit GH, Tobin C, Krishnan K, Moricard Y, Covey DF, Rondi-Reig L, Akwa Y, 2011. Pregnenolone sulfate and its enantiomer: differential modulation of memory in a spatial discrimination task using forebrain NMDA receptor deficient mice. Eur. Neuropsychopharmacol 21, 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Engler-Chiurazzi EB, Lewis SE, Rath NP, Simpkins JW, Covey DF, 2016. Structure-activity studies of non-steroid analogues structurally-related to neuroprotective estrogens. Org. Biomol. Chem 14, 9790–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Krishnan K, Kudova E, Li P, Manion BD, Taylor A, Elias G, Akk G, Evers AS, Zorumski CF, Mennerick S, Covey DF, 2014Q. Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of β-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J. Med. Chem 57, 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikes AC, Hernandez GD, Matthews DC, Lukic AS, Law M, Shi Y, Schneider LS, Brinton RD, 2021. Exploratory imaging outcomes of a phase 1b/2a clinical trial of allopregnanolone as a regenerative therapeutic for Alzheimer’s disease: structural effects and functional connectivity outcomes. Alzheimer’s Dement 8, e1225B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA, 2016. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol. Sci 37, 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers-Neame NT, Covey DF, Hu Y, Isenberg KE, Zorumski CF, 1992. Effects of a benz[e]indene on gamma-aminobutyric acid-gated chloride currents in cultured postnatal rat hippocampal neurons. Mol. Pharmacol 42, 952–957. [PubMed] [Google Scholar]
- Rudajev V, Novotny J, 2022. Cholesterol as a key player in amyloid β-mediated toxicity in Alzheimer’s disease. Front. Mol. Neurosci 15, 937056 10.3389/fnmol.2022.937056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed K, Jo MH, Park JS, Alam SI, Khan I, Ahmad R, Khan A, Ullah R, Kim MO, 2021. 17β-Estradiol abrogates oxidative stress and neuroinflammation after cortical stab wound injury. Antioxidants 10, 1682. 10.3390/antiox10111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seljeset S, Bright DP, Thomas P, Smart TG, 2018. Probing GABAA receptors with inhibitory neurosteroids. Neuropharmacol 136, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear DA, Galani R, Noffman SW, Stein DG, 2002. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp. Neurol 178, 59–67. [DOI] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF, 2000. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J. Neurosci 20, 3571–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H-J, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S, 2004. Slow actions of neuroactive steroids at GABAA receptors. J. Neurosci 24, 6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Richardson TE, Yi KD, Perez E, Covey DF, 2013. Neuroprotection with non-feminizing estrogen analogues: an overlooked possible therapeutic strategy. Horm. Behav 63, 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, 2013. The influence of stress at puberty on mood and learning: role of the β4β GABAA receptor. Neuroscience 249, 192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneitz N, Krishnan K, Covey DF, Finel M, 2011. Glucuronidation of the steroid enantiomers ent-17β-estradiol, ent-androsterone and ent-etiocholanolone by the human UDP-glucuronosyltransferases. J. Steroid Biochem. Mol. Biol 127, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa Y, Cheng WWL, Bracamontes JR, Chen Z-W, Wang L, Germann AL, Pierce SR, Senneff TC, Krishnan K, Reichert DE, Covey DF, Akk G, Evers AS, 2020. Site-specific effects of neurosteroids on GABAA receptor activation and desensitization. eLife 9, e55331. 10.7554/eLife.55331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhu H, Clark S, Gouaux E, 2023. Regulated assembly and neurosteroid modulation constrain GABAA receptor pharmacology in vivo. bioRxiv. 10.1101/2023.02.16.528867. February 16, 2023. [DOI] [Google Scholar]
- Tateiwa H, Chintala SM, Chen Z, Wang L, Amtashar F, Bracamontes J, Germann AL, Pierce SR, Covey DF, Akk G, Evers AS, 2023. The mechanism of enantioselective neurosteroid actions on GABAA receptors. Biomolecules 13, 341 https://doi.org. 10.3390/biom13020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L, 1968. Differential inhibition in vitro of 17beta-estradiol binding in the mouse uterus and vagina by optical antipodes of estrogens. Mol. Pharmacol 4, 301–310. [PubMed] [Google Scholar]
- Thomas P, Pang Y, 2020. Anti-apoptotic actions of allopregnanolone and ganaxolone mediated through membrane progesterone receptors (PAQRs) in neuronal cells. Front. Endocrinol 11, 417. 10.3389/fendo.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, Covey DF, Lingle CJ, 1998. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks gamma-aminobutyric acid-modulatory activity. Mol. Pharmacol 54, 918–927. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF, 2011. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J. Neurosci 31, 9905–9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF, 2010. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J. Neurosci 30, 16788–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Shen W, Heinrichs SC, Zorumski CF, Covey DF, Koob GF, Purdy RH, 2001. Steroid structure and pharmacological properties determine the anti-amnesic effects of pregnenolone sulfate in the passive avoidance task in rats. Eur. J. Neurosci 14, 2003–2010. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, Hammes SR, Jamnongjit M, Stein DG, 2006. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology 51, 1078–1085. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Suber J, Lewandowski M, 2013. US 8,492,368 B2, Nasal delivery mechanisms for prophylactic and post-acute use of progesterone and/or its enantiomer for use in treatment of mild traumatic brain injuries. July 23, 2013.
- Vien TN, Ackley MA, Doherty JJ, Moss SJ, Davies PA, 2022. Preventing phosphorylatio of the GABAAR β3 subunit compromises the behavioral effects of neuroactive steroids. Front. Mol. Neurosci 15, 8173389. 10.3389/fnmol.2022.817996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitku J, Hill M, Kolatorova L, Havrdova EK, Kancheva R, 2022. Steroid sulfation in neurodegenerative disorders. Front. Mol. Biosci. 9, 839887 10.3389/fmolb.2022.839887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler A, Wehling M, 2022. Many or too many progesterone membrane receptors? Clinical implications. Trends Endocrinol. Metab 33, 850–868. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF, 2004. The enantiomer of cholesterol. J. Membr. Biol 202, 61–72. [DOI] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF, 1996. Enantioselectivity of steroid-induced gamma-aminobutyric acidA receptor modulation and anesthesia. Mol. Pharmacol 50, 1581–1586. [PubMed] [Google Scholar]
- Wnuk A, Kajta M, 2017. Steroid and xenobiotic receptor signaling in apoptosis and autophagy of the nervous system. Int. J. Mol. Sci 18, 2394. 10.3390/ijms18112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, 2007. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol 47, 657–680. [DOI] [PubMed] [Google Scholar]
- Yi KD, Cai ZY, Covey DF, Simpkins JW, 2008. Estrogen receptor independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J. Pharmacol. Exp. Ther 324, 1188–1195. [DOI] [PubMed] [Google Scholar]
- Yi KD, Covey DF, Simpkins JW, 2009. Mechanism of okadaic-acid induced neuronal death and the effect of estrogens. J. Neurochem 108, 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri S, Mellon SH, Butters TD, Nevyjel M, Covey DF, Bembi B, Dardis A, 2009. Oxidative stress in NPC1 deficient cells: protective effects of allopregnanolone. J. Cell. Mol. Med 13, 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Zhang Y, Ma J, Xu J, 2015. Progesterone for acute traumatic brain injury: a systematic review of randomized controlled trials. PLoS One 10, e0140624. 10.1371/journal.pone.0140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowski D, Dhir A, Krishnan K, Covey D, Rogawski MA, 2014. Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms. Psychopharmacol 231, 3325–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y, 2012Z. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev 36, 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Covey DF, 1998. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse 29, 162–171. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Covey DF, Mennerick S, 2019. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress 11, 100196. 10.1016/j.ynstr.2019.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S, 2013. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev 37, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Wittmer LL, Isenberg KE, Hu Y, Covey DF, 1996. Effects of neurosteroid and benz[e]indene enantiomers on GABAa receptors in cultured hippocampal neurons and transfected HEK-293 cells. Neuropharmacol 35, 1161–1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.