Abstract
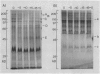
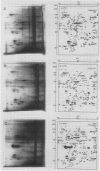
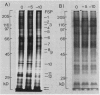
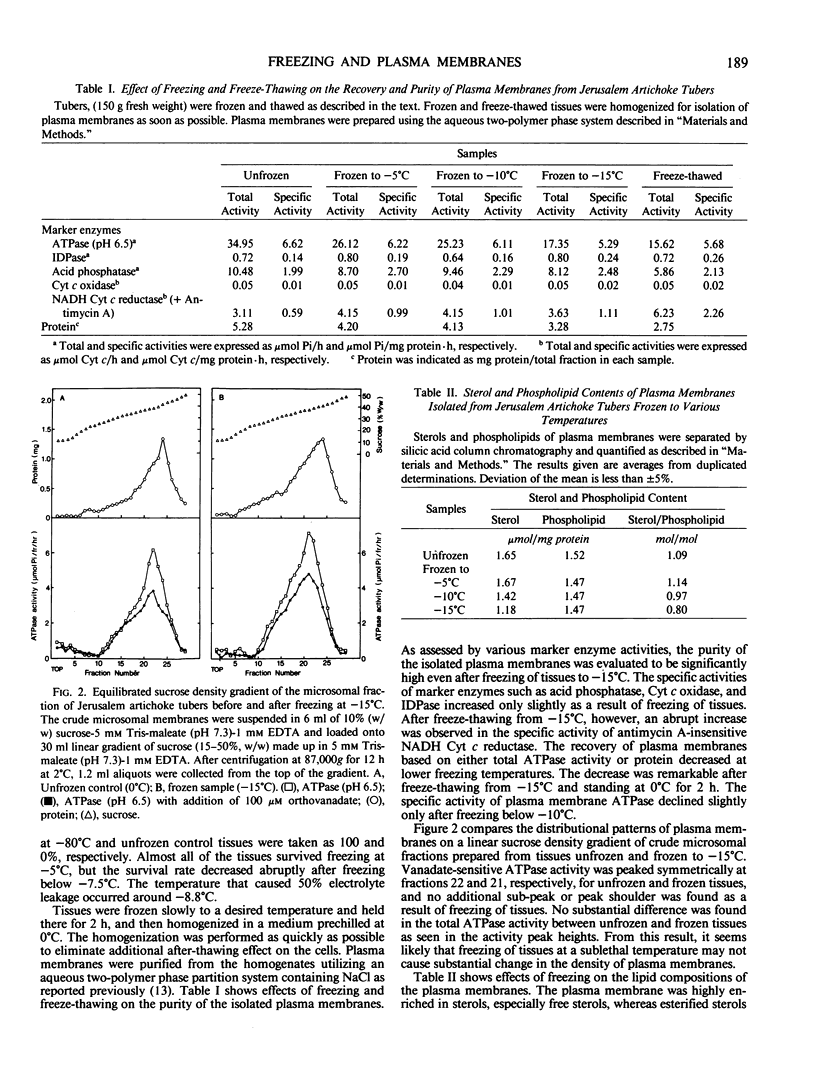
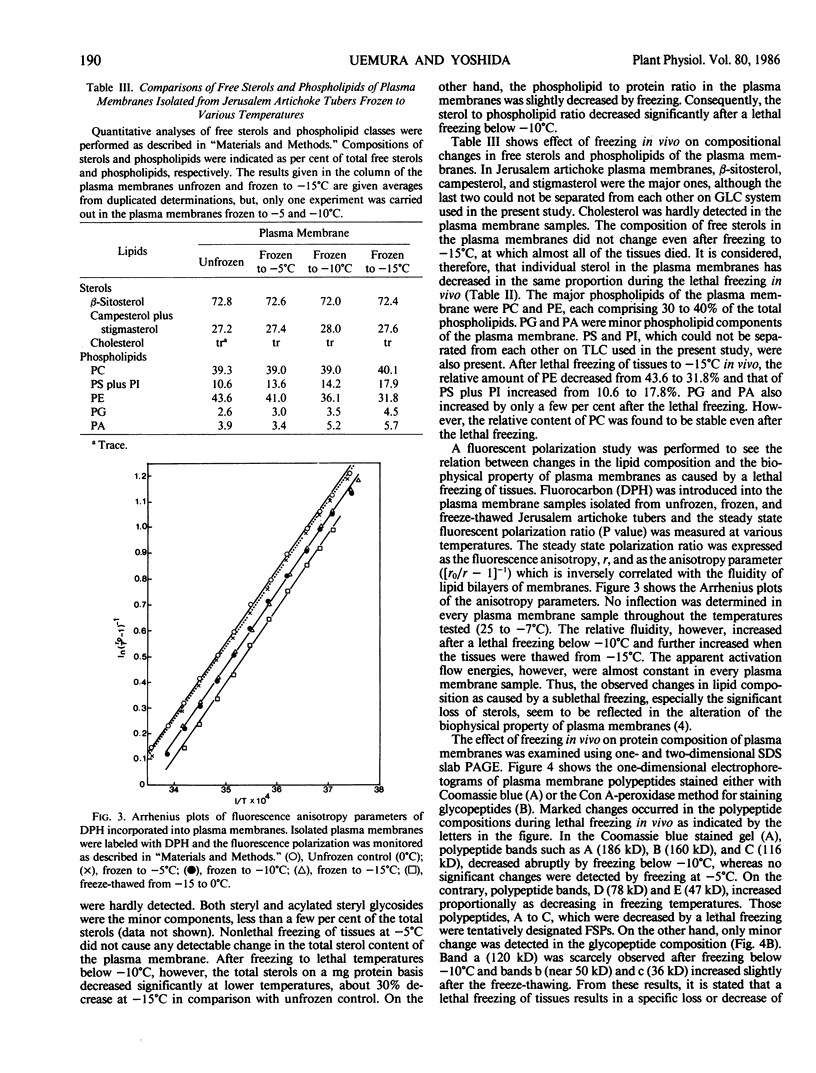
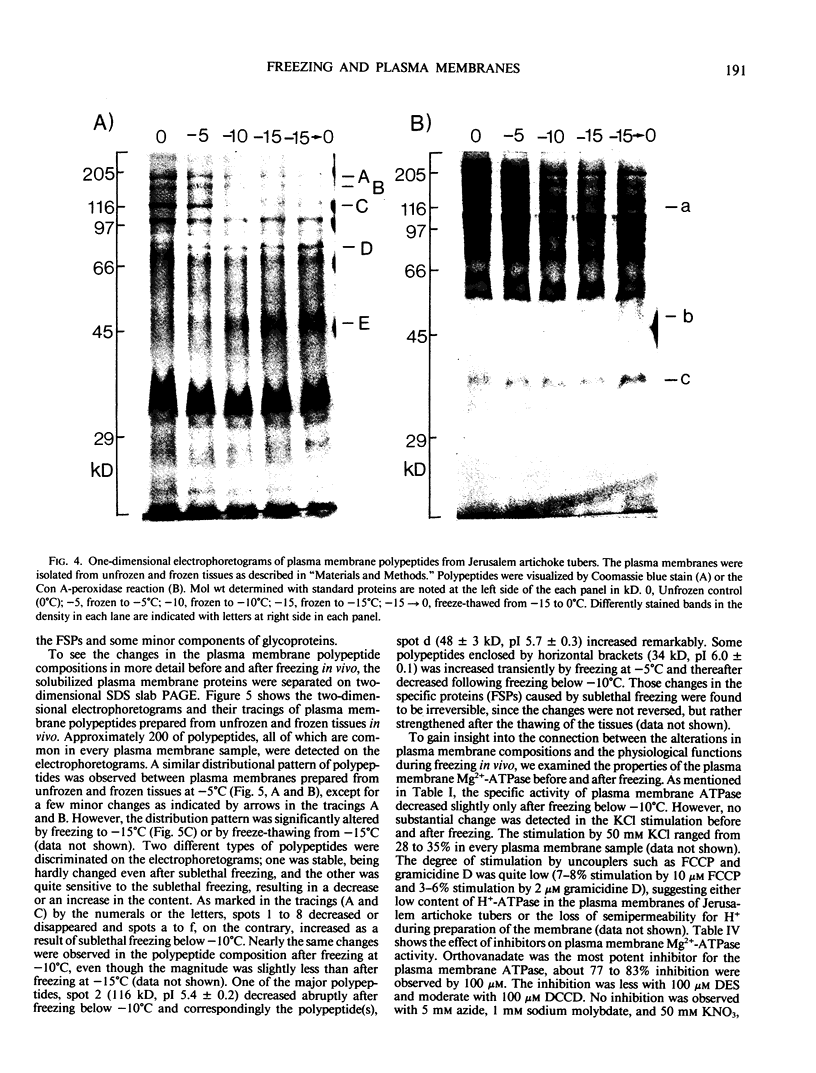
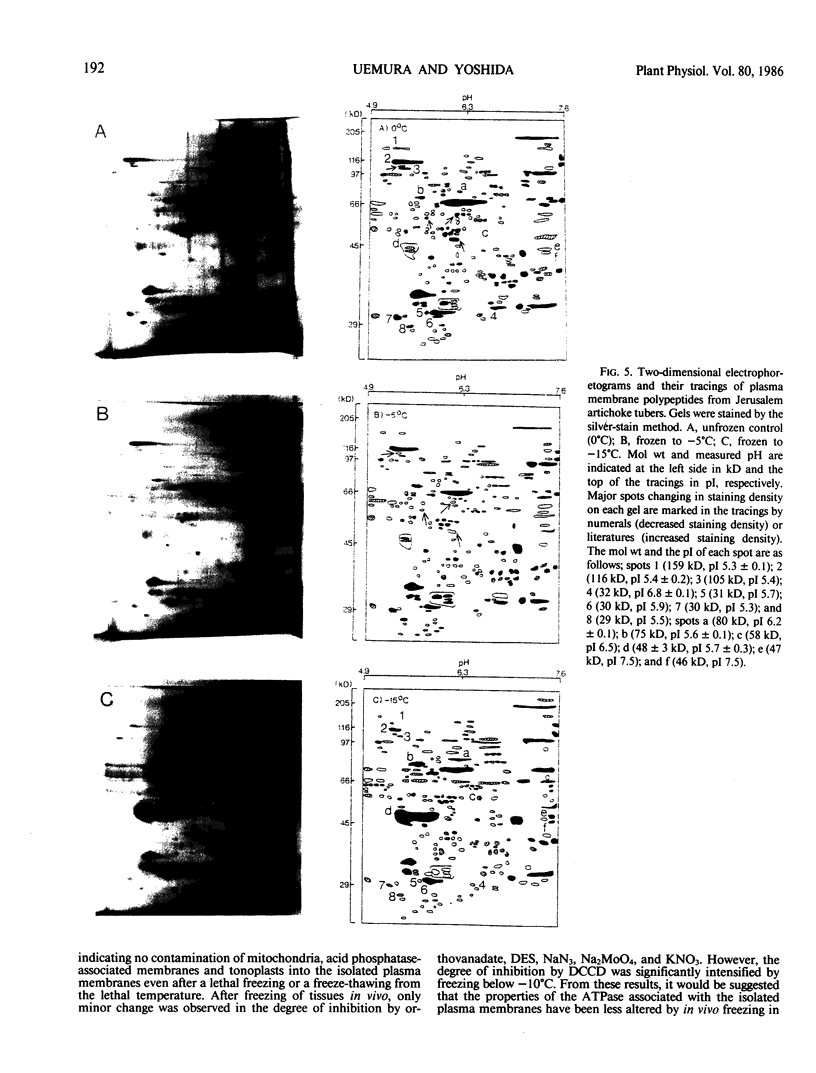
Plasma membranes were isolated from both unfrozen and frozen tissues of Jerusalem artichoke tubers (Helianthus tuberosus L.) in high purity utilizing an aqueous two-polymer phase partition system. Although the recovery of the plasma membranes was decreased significantly by freezing of tissues even at the nonlethal temperature (−5°C), the isolated plasma membrane samples were considered to be representative of the plasma membranes in situ. Freezing of the tissues at sublethal temperatures resulted in marked changes in the chemical composition of the plasma membrane. Those are losses of sterols and phosphatidylethanolamine from the plasma membranes, and a change of specific proteins with relatively high molecular weights into low molecular weight peptides. These specific proteins were designated as frost susceptible proteins. The properties of the plasma membrane ATPase seem to be not affected so much by the in vivo freezing of cells. However, inhibition of the plasma membrane ATPase by N,N′-dicyclohexylcarbodiimide (DCCD) was relatively low before and after freezing in vivo at the nonlethal temperature at −5°C, but was markedly enhanced by freezing in vivo at sublethal temperatures below −10°C. From the results, it is assumed either that the enzyme molecule was partially modified, especially at the presumed DCCD binding sites or that the DCCD had become more accessible to the enzyme as a result of increased permeability of the plasma membranes. These observed changes are discussed in connection with the mechanism of cell injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki T. Release of cholesterol-enriched microvesicles from human erythrocytes caused by hypertonic saline at low temperatures. FEBS Lett. 1979 Jan 15;97(2):237–240. doi: 10.1016/0014-5793(79)80092-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Alterations in Chloroplast Thylakoids during an in Vitro Freeze-Thaw Cycle. Plant Physiol. 1976 May;57(5):673–680. doi: 10.1104/pp.57.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Steponkus P. L. Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6373–6377. doi: 10.1073/pnas.81.20.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Parish R. W., Schmidlin S., Müller U. The effects of proteases on proteins and glycoproteins of Dictyostelium discoideum plasma membranes. Exp Cell Res. 1977 Dec;110(2):267–276. doi: 10.1016/0014-4827(77)90292-0. [DOI] [PubMed] [Google Scholar]
- Quinn P. J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology. 1985 Apr;22(2):128–146. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- Singh J., Miller R. W. Spin-Probe Studies during Freezing of Cells Isolated from Cold-Hardened and Nonhardened Winter Rye : MOLECULAR MECHANISM OF MEMBRANE FREEZING INJURY. Plant Physiol. 1982 Jun;69(6):1423–1428. doi: 10.1104/pp.69.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Dowgert M. F., Gordon-Kamm W. J. Destabilization of the plasma membrane of isolated plant protoplasts during a freeze-thaw cycle: the influence of cold acclimation. Cryobiology. 1983 Aug;20(4):448–465. doi: 10.1016/0011-2240(83)90035-4. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Studies on Freezing Injury of Plant Cells: I. Relation between Thermotropic Properties of Isolated Plasma Membrane Vesicles and Freezing Injury. Plant Physiol. 1984 May;75(1):38–42. doi: 10.1104/pp.75.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]