Abstract
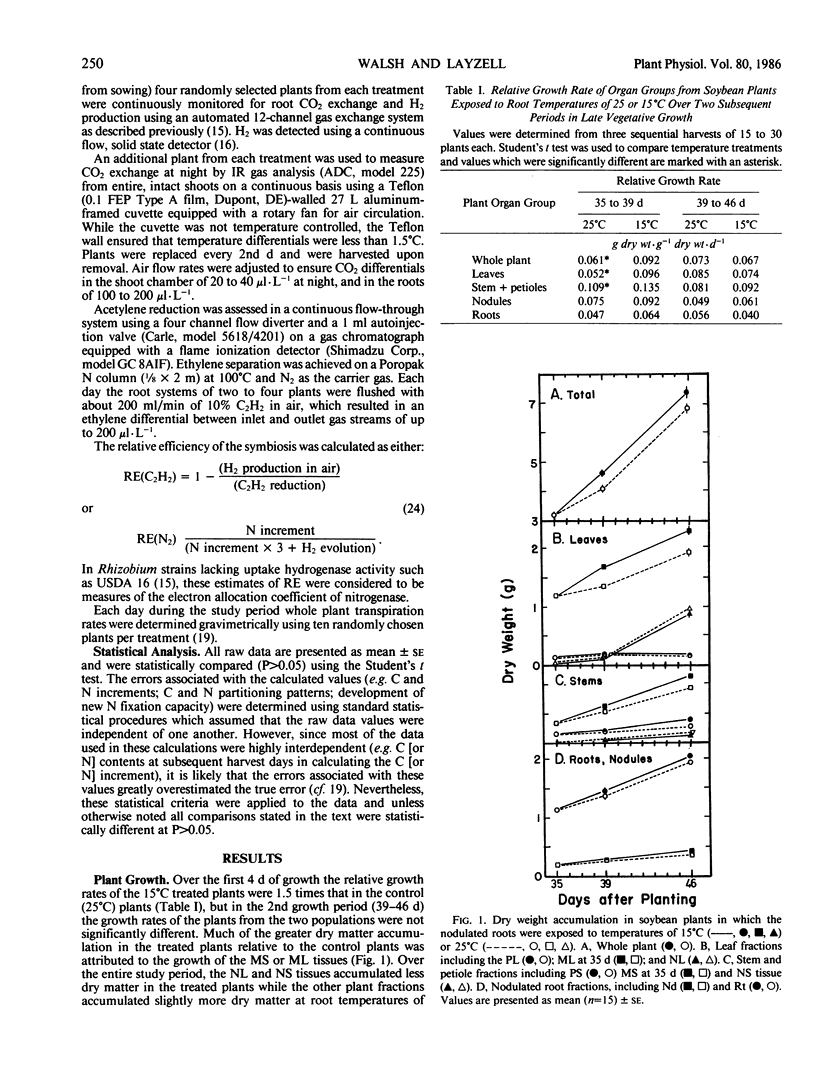
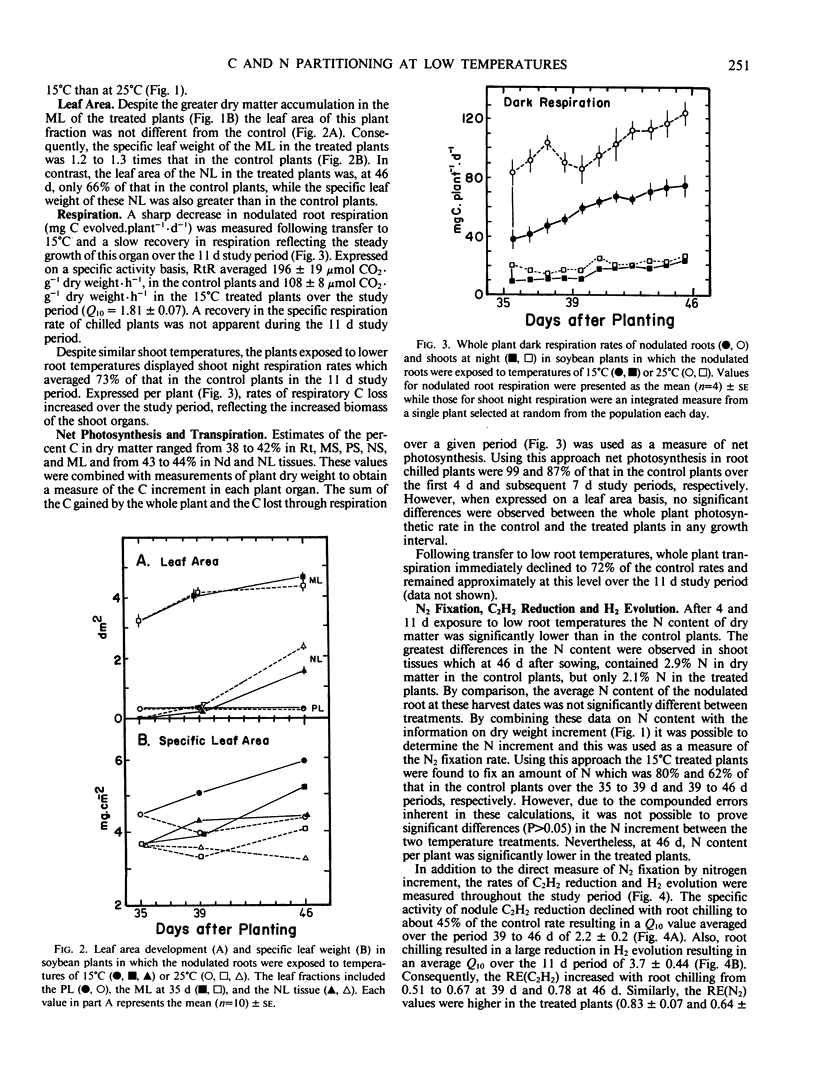
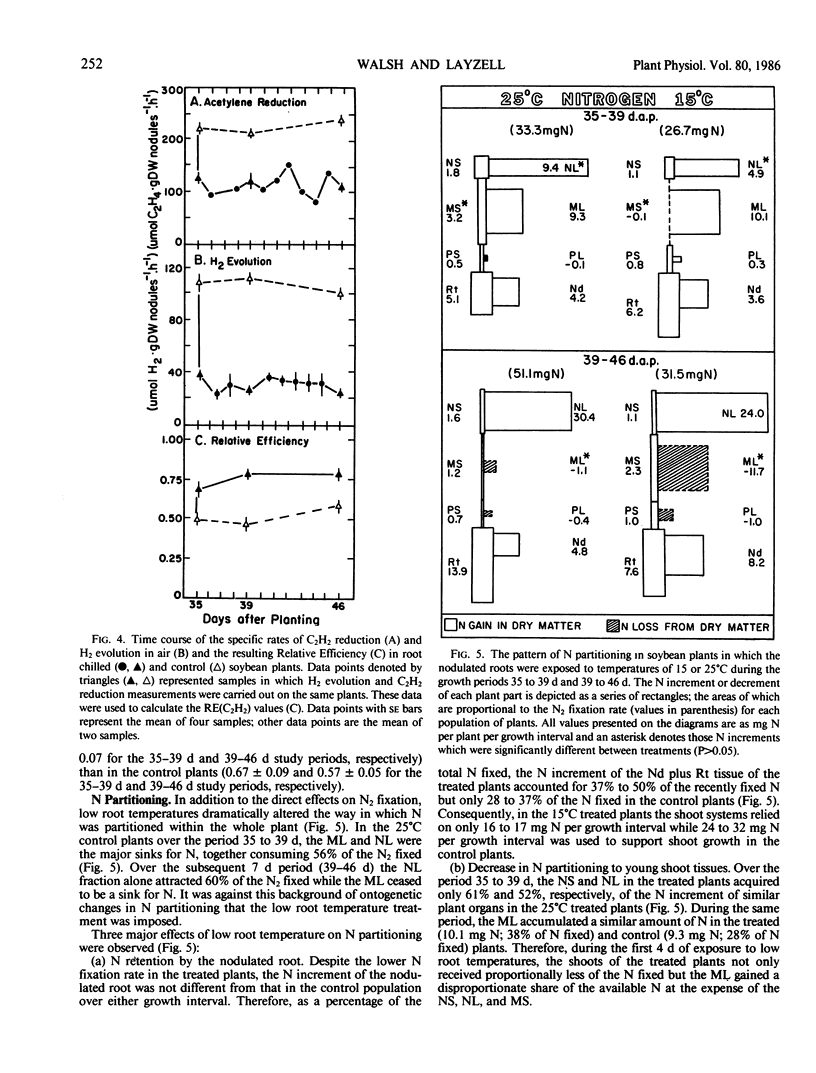
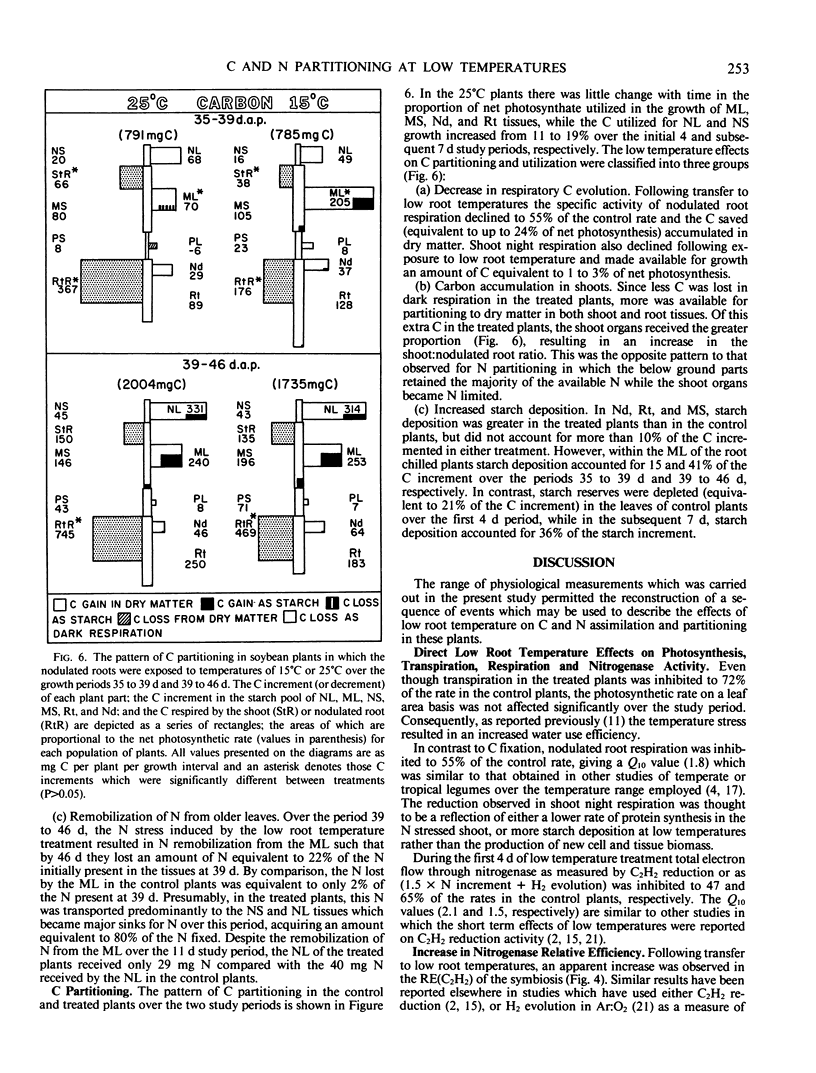
Low root temperature effects on vegetative growth of soybean (Harosoy 63 × Rhizobium japonicum USDA 16) were examined in 35 day old plants exposed to temperatures of 15°C (shoots at 25°C) for an 11 day period. Duing this period various aspects of C and N assimilation and partitioning were monitored including shoot night and nodulated root respiration, C and N partitioning to six plant parts, C2H2 reduction, H2 evolution, leaf area, transpiration, net photosynthesis, and N2 fixation. The low temperature treatment resulted in a decrease in the net rate of N2 fixation but nitrogenase relative efficiency increased. In response, the plant retained N in the tissues of the nodulated root and decreased N partitioning to young shoot tissues, thereby inducing the remobilization of N from older leaves, and reducing leaf area development. The leaf area specific rate of net photosynthesis was not affected over the study period; however, shoot and nodulated root respiration declined. Consequently, C accumulated in mature leaves and stems, partly in the form of increased starch reserves. Three possibilities were considered for increasing low temperature tolerance in nodulated soybeans: (a) decrease in temperature optima for nitrogenase, (b) increased development of nodules and N2 fixation capacity at low temperature, and (c) alterations in the pattern of C and N partitioning in response to low temperature conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Bertelsen H. Effect of temperature on h(2) evolution and acetylene reduction in pea nodules and in isolated bacteroids. Plant Physiol. 1985 Feb;77(2):335–338. doi: 10.1104/pp.77.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon J. J., Frazier L., Russell S. A., Evans H. J. Respiratory and Nitrogenase Activities of Soybean Nodules Formed by Hydrogen Uptake Negative (Hup) Mutant and Revertant Strains of Rhizobium japonicum Characterized by Protein Patterns. Plant Physiol. 1982 Nov;70(5):1341–1346. doi: 10.1104/pp.70.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Henson C. A., Servaites J. C., Vogelzang R. D., Pendleton J. W. Low root temperature effects on soybean nitrogen metabolism and photosynthesis. Plant Physiol. 1979 May;63(5):956–962. doi: 10.1104/pp.63.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edie S. A., Phillips D. A. Effect of the host legume on acetylene reduction and hydrogen evolution by Rhizobium nitrogenase. Plant Physiol. 1983 May;72(1):156–160. doi: 10.1104/pp.72.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Electron allocation to alternative substrates of Azotobacter nitrogenase is controlled by the electron flux through dinitrogenase. Biochim Biophys Acta. 1980 Jun 10;591(1):63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- Layzell D. B., Weagle G. E., Canvin D. T. A highly sensitive, flow through h(2) gas analyzer for use in nitrogen fixation studies. Plant Physiol. 1984 Jul;75(3):582–585. doi: 10.1104/pp.75.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., McNeil D. L. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume. Plant Physiol. 1979 Apr;63(4):730–737. doi: 10.1104/pp.63.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A., Pate J. S. Effect of temperature on nitrogenase functioning in cowpea nodules. Plant Physiol. 1983 Oct;73(2):392–394. doi: 10.1104/pp.73.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R. Nitrogenase of Klebsiella pneumoniae. Distinction between proton-reducing and acetylene-reducing forms of the enzyme: effect of temperature and component protein ratio on substrate-reduction kinetics. Biochem J. 1977 Nov 1;167(2):457–461. doi: 10.1042/bj1670457. [DOI] [PMC free article] [PubMed] [Google Scholar]


