Summary
Cannabinoid CB1 receptors have been shown to regulate wide array of functions ranging from homeostasis to the cognitive functioning but recent data support the hypothesis that astrocytes also operate as a mediator of synaptic plasticity and contribute to cognition and learning. The receptor heterogeneity plays a key role in understanding the molecular mechanisms underlying these processes. Despite the fact that the majority of CB1 receptors act on neurons, studies have revealed that cannabinoids have direct control over astrocytes, including energy generation and neuroprotection. The tripartite synapse connects astrocytes to neurons and allows them to interact with one another and the astrocytes are key players in synaptic plasticity, which is associated with cognitive functions. This review focuses on our growing understanding of the intricate functions of astroglial CB1 that underpin physiological brain function, and in Alzheimer's disease.
Keywords: Alzheimer's disease, Cannabinoid, Astrocytes, Cognition
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder with cognitive and memory impairments. Pathological deposition of amyloid-β peptides, hyper phosphorylation of tau as paired helical filaments and neurofibrillary tangles were all reported, along with synaptic dysfunction, neuronal loss, enhanced neuroinflammation, and oxidative stress [1]. In turn, inflammation and oxidative stress also contribute to AD progression [2]. Astroglia have long been viewed as a passive and supportive cells, while microglia have recently received a lot more attention and are recognized to be implicated in the neuroinflammation of AD [3,4]. Recent evidence suggests that astrocytes play a paramount role in the progression of Alzheimer's disease (Table 1) [5,6,7]. In astrocytes a large number of genes are expressed, and more than 40 genetic loci have been linked to the risk of Alzheimer's disease. Endocytosis, metabolism, and inflammation have all been identified as new potential therapeutic targets in genome-wide association studies (GWAS) [8]. Interestingly, a recent transcriptome analysis revealed that in AD mice, 33 age-up genes were upregulated in astrocytes, while 53 age-down genes were downregulated [9].
Table 1.
Molecular targets in astrocytes and astrocyte communication that are altered in AD.
| Impaired | Outcome | Reference |
|---|---|---|
| GLUT1 | Metabolic dysregulation | [23] |
| Gap junctions/hemichannels | Homeostasis of CNS | [24] |
| Glutamate receptor | Excitotoxicity | [25] |
| Glutamate transporter (GLT1) | Excitotoxicity | [26] |
| Calcium signaling | Cognitive dysfunctions | [27,28] |
| Purinergic signaling | Cognitive dysfunctions | [29] |
| JAK-STAT3 | Disrupted astrogliosis | [30,31] |
| MAPK | Inflammation | [32] |
| NRF2 | Oxidative stress | [33] |
| TNF | Inflammation | [34] |
Morphobiology of astrocytes
Astrocytes are star-shaped cells with branches that have specialized structures at the end of their processes called “end feet”. These are also known to regulate a variety of functions in the central nervous system (CNS).
Astrocyte heterogeneity
Based on their morphology and location astrocytes were previously classified as protoplasmic or fibrous. The former is found in grey matter and contains fine, evenly structured processes, whereas the latter is found in white matter and has few branching processes that are oriented longitudinally. Müller glia in the retina, Bergmann glia in the cerebellum, marginal glia, radial, ependymal, perivascular glia, velate, and tanycytes are some of the specialized astrocytes that have been classified for further clarification [10,11], and all these heterogenous astrocytes together termed as astroglia [8]. Later in nineteenth century two different classes of non-neuronal cells, the oligodendrocytes and microglia were identified. Thus, the current concept of central glia emerged, which includes microglia, oligodendrocytes, and astrocytes. More recently, NG2 cells (oligodendrocyte progenitor) were discovered based on the expression of chondroitin sulphate proteoglycan, which may include the fourth category of central glia [11].
The type of stimuli that activate astrocytes differs. For example, inflammation activates A1 astrocytes, which are neurotoxic, while ischemia activates A2 astrocytes, which are protective. Atrophic astrocytes and reactive astrocytes are the two types of astrocytes that are traditionally classified. Atrophic astrocytes have a lower volume and fewer processes, whereas reactive astrocytes have a larger volume and thicker processes [6]. The cytoarchitecture of astrocytes differs in vitro and in vivo, with cultured astrocytes having fewer ramifications than in vivo [12]. These glial cells undergo morphological and functional alterations under pathological conditions, transforming them into reactive astrocytes. These reactive astrocytes express certain cytoskeletal component proteins such as glial fibrillary acidic protein (GFAP), vimentin, nestin [13]. Furthermore, these the reactive astrocyte cells also known to express increased levels of the enzyme monoamine oxidase-B (MAO-B) [6].
Characteristic of astrocytes
A complex, diverse larger astrocytes are generally found in humans. The main structural features of astrocytes include the presence of gap junctions due to connexins, filament bundles, glycogen granules, astrocyte extensions around blood vessels, synapses, presence of the aquaporins, receptors and the ion channels while, the main functional features include the non-excitability, high negative membrane potential, cell signaling mediated by Ca2+ ions, neurotransmitter uptake by various transporters. Astrocytes regulate energy metabolism, homeostasis, inflammatory axis, and various signaling pathways involving calcium signaling, purinergic signaling, Jak-Stat3, MAPK, TGF-β, cholesterol production and in growth factor (IGF-1, BDNF, CCL5, NGF) functions [10,14]. Thus, astrocytes play a crucial role to preserve neurological function. Any changes in their physiological functions may lead to various neurological diseases. One such disease is Alzheimer's disease (AD).
Astrocyte functions
Astrocytes perform more functions than any other sort of nervous system cell. These astrocytes form the scaffold for the entire nervous system and occupy a huge amount of the space of the CNS and form the majority of the structure that comprises the brain and spinal cord. They provide structural support as well as a home for all other cells, such as neurons and glia [3,10,15]. If the CNS is injured, these astrocytes proliferate and generate more astrocytes, which migrate to the affected location and undergo hypertrophy, where they develop into much larger, thicker, and longer processes that surround the damage and form a thick layer of scar tissue between them [10,15]. The entire process of astrocytes reacting to injury is termed as gliosis/astrogliosis/astrocytosis/reactive astrocytosis, and the scar tissue formed is known as a glial scar. Astrocytes also help in maintenance of homeostasis, specifically the homeostasis of the interstitial fluid, which is critical since neurons require a very finely tuned, even-keeled environment to function effectively. Astrocytes constantly monitor the interstitial fluid to keep ion concentrations at their optimal levels (K+ buffering), as well as release lactate and provide energy to neurons [15]. The contribution of astrocytes to the blood-brain barrier is another key role. Astrocytes attach their feet to the blood vessels that flow through the CNS, preventing numerous big molecules from exiting the bloodstream and entering the brain. The clearing out of synapses between neurons is another important function of astrocytes; it is vital to reset the synapse so that it can be used for communication again. Besides these functions, astrocytes have an effect on neurons and other glial cells, and vice versa, through the exchange of various chemicals [10].
Astrocyte communications
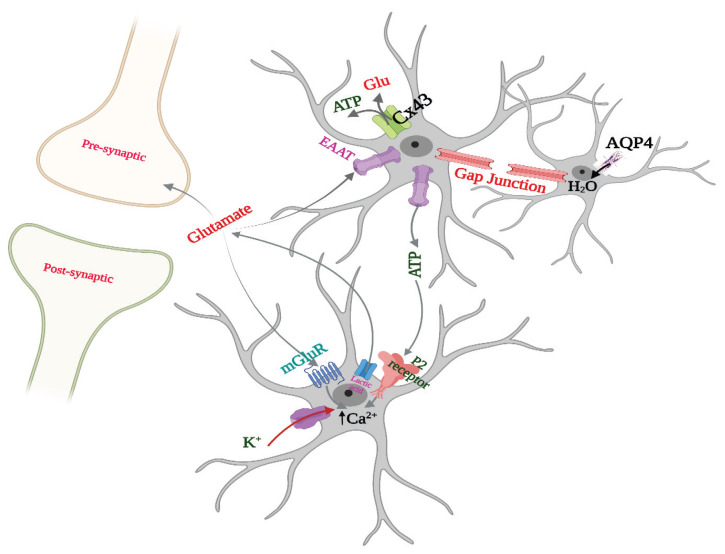
Gap junctions and purine signaling are used by astrocytes to communicate with other astrocytes, emphasizing astrocyte communication in the brain. Apart from direct communication via gap junctions, astrocytes use hemichannels to send signals to neighboring neurons, microglia, and oligodendrocytes, allowing for crosstalk. Astrocytes communicate with pre- and post-synaptic neurons, forming a tripartite synapse (a communication between pre- and post-synaptic neurons and astrocyte processes) (Fig. 1), and interact intercellularly via glutamate and ATP release via hemichannels (connexin 43), modulating synaptic function [10]. The peri synaptic astrocyte processes (PAPs) are the thin membranes surrounding synapses, and these express some lamellar proteins such as glutamate transporters (EAAT), neurotransmitter machinery, cholinergic receptors (α7nicotinic, muscarinic) while the post-synaptic astrocytes express CB1 cannabinoid, purinergic, TNF receptors, and the lactate metabolite transporter (MCT1). Apart from these, astrocytes release different molecules that act on synapses such as neurotransmitters: glutamate, GABA, acetylcholine, ATP and adenosine [16], neuromodulators like D-serine [17], purines [18], metabolic substances such as lactate, and tissue hormones such as growth factors, TNF-α [19], which affect different facets of plasticity [20] and this process is termed as gliotransmission [21]. Gap junction coupling between adjacent glial cells is mediated by astrocytic-astrocyte extensive connections, which form a cellular network known as the astrocytic 'syncytium' [15]. Astrocytes detect neural activity and react by increasing intracellular Ca2+ levels and releasing transmitters. The hippocampus CA1 LTP, for example, requires D-serine, which is released by astrocytes. The release of D-serine raises NMDAR occupancy to a threshold level, triggering the downstream processes necessary for LTP induction [22,10]. Astrocytes also express a variety of pumps and transporters, including the GLUT1 and MCT1 transporters, which deliver energy to astrocytes and neurons [20]. The astrocyte has a lot of potassium (Kir 4.1) and calcium channels that help with ion influx and efflux across the membrane. GLT-1 and GLAST are astrocyte-specific glutamate transporters that contribute in the rapid clearance of neurotransmitters produced into the synaptic cleft [35]. Astrocytes communicate with blood-brain barrier through the water channel aquaporin 4 (AQP4) which is required for the regulation of cell volume (Fig. 1) [10].
Fig. 1.
Astrocyte physiological functions and the formation of tripartite synapse with intercellular communications. Arrows in black indicates facilitatory affect. The image was created on Biorender.com under paid subscription.
Introduction to the endocannabinoid system
Cannabinoid receptors (CBR) are seven-transmembrane spanning metabotropic receptors that attach to tetrahydro cannabinol (THC), the main psychotropic component in marijuana. These CBR bind to endogenous ligands called endocannabinoids (eCBs), which include anandamide and 2-AG. They are the signalling molecules that maintain homeostasis after pathogenic insults. The CNR1 and CNR2 genes, which have been mapped to human chromosomes 6q14–15 and 1p36, encode the CB1 and CB2 receptors [36,37].
CB1R is located mainly in the cingulate gyrus, hippocampus, cerebellum, cortex, and basal ganglia [38]. CB2R are found in the spleen, circulating immune cells macrophage-derived cells including microglia, astrocytes, tonsils, osteocytes, liver cells and also found in hippocampus, entorhinal cortex, hypothalamus, thalamus, striatum [4,39].
Endocannabinoids like anandamide (AEA) and 2-arachidonylglycerol (2-AG) are synthesized in the body under physiological conditions [40,41]. AEA shows high affinity for CB1R while 2-AG exerts moderate to low affinity for both receptor subtypes. AEA acts as a partial agonist at CB1R while 2-AG acts as a full agonist for both receptors [42].
Dual role of astrocytes in generation and elimination of amyloid-β peptides
Astrocytes play a bidirectional role in the production and clearance of amyloid-β peptide, and the astroglial dysfunction contributes directly to the pathogenesis of AD [12]. Increased oxidative stress and the generation of reactive oxygen species can trigger detrimental pathways in astrocytes, while reactive astrocytes that were supposed to eliminate or degrade β-amyloid become distorted and contribute to Aβ aggregation. Although astrocytic Aβ production is lower than that of neurons, due to their greater number, astrocytic Aβ production is known to have a significant impact on AD progression. The reactive astrocytes release number of inflammatory cytokines.
The inhibitory gliotransmitter GABA's uncontrolled release impairs hippocampus memory functions, whereas the astrocyte's inability to control the excitatory transmitter glutamate causes a loss of balance in frontal brain function [36]. In Alzheimer's disease, pro-inflammatory cytokines are increased, and initial defensive inflammatory responses by reactive astrocytes against Aβ plaque development gradually contribute to amyloid load as a result of cellular and molecular stresses causing enzymatic alterations inside microglia and astrocytes [37]. Interferon-γ, TNF-α, and IL-1β treatment of primary mouse astrocytes resulted in enhanced astrocytic production of amyloid precursor protein, as well as BACE1 and γ-secretase [36]. Hence, the levels of AD biomarkers were progressively elevated due to exposure to pro-inflammatory factors, indicating that sustained exposure to inflammatory mediators leads to not only metabolic dysfunction but also increased amyloid expression [38]. In contrast, Astrocytes help in the clearance of amyloid plaques via phagocytic and enzymatic pathways. In response to the presence of monocyte chemoattractant protein 1 (MCP1) in the lesions, astrocytes bind to Aβ proteins and elevate calcium and magnesium divalent ion exposure [39]. The presence of additional binding molecules on Aβ, such as the receptor for advanced glycation end products (RAGE), proteoglycans, and low-density lipoprotein receptors, can cause astrocytes and amyloid-β to bind [38,39].
Cannabinoid receptors and signaling in astrocytes
The endocannabinoid system in astrocytes is fully functional, with the ability to produce and inactivate endocannabinoids. CB1 receptors are found in the spinal cord [40], the hippocampus [41], the neocortex [42], and the caudate putamen [43]. Mostly cannabinoid 1 receptors are expressed in the neurons [44], microglia [4] and other glial cells. CB1receptors in the brain mediate a variety of metabolic and cellular events [45], influencing metabolic and behavioral activities, as evidenced by research from knock-out mice [46].
The endocannabinoid signaling involves a retrograde pathway in which the endocannabinoid mobilized from the post synaptic terminal activates the presynaptic CB1 receptors [47], which inhibit calcium channels (voltage-gated), hence limiting neurotransmitter release [48].
Because CB1 receptors are GPCRs, they are activated by ligand binding [49]. In astrocytes, these receptors are coupled to Gq/11 G-proteins, which activate the phospholipase C and diacyl glycerol pathway, generating inositol 1,4,5-trisphosphate and calcium mobilization from intracellular stores. This activation of CB1R elevates astrocyte Ca2+ concentrations, which evoke glutamate-mediated signaling in tripartite synapse [47].
Calcium signaling independent of the CB1R has also been proposed, which is known to be carried out by the transient receptor potential A1 (TRPA1) channels and reduces GABA release and D-serine [50]. Even though this process is not dependent on CB1R, it is modulated by endocannabinoids [45], and the cannabinoid system activates to protect neurons from excitotoxic injury by acting “on demand” [51].
The slender perisynaptic astrocytic processes engage physically with synapses in an active manner that is influenced by activity-induced plasticity signals, implying the role of astrocytes in physical synapse stabilization [15]. Because of the high K+ concentrations, astrocytes have a negative resting membrane potential and a high K+ permeability. The coupled astrocyte syncytium has “isopotentiality” due to its high baseline K+ conductance and extensive gap junctional coupling, which reduces activity-induced astrocyte membrane potential variations [52].
Astrocytes serve as a bridge for inter neuronal communication [47] and the cannabinoid mediated signaling plays an essential role in learning and memory. Gliotransmission involves various events that occur when the astrocytes release different bioactive molecules in a calcium dependent manner. Following depolarization, synaptic depression is induced and termed as the depolarization-induced suppression of excitation (DSE) in glutamatergic synapses [53] while in the GABAergic synapses it is depolarization-induced suppression of inhibition (DSI) [45].
Endocannabinoids activate the CB1 receptors during neuronal depolarization, which is crucial for resetting the synapse so that it can be used for communication again. Finally, astrocytes assist in the release of a neurotransmitter that are distant from the depolarized neurons. Thus, endocannabinoids activate a long-range signal involving astrocyte calcium mobilization and glutamate release, which results in synaptic potentiation of distant synapses indirectly. This process is known as lateral synaptic regulation, and it was initially discovered in the hippocampus [54].
The released glutamate activates CA1 pyramidal neurons as well as inhibitory GABAergic neurons when the Schaffer collaterals are activated. GABA activates GABA-B receptors in astrocytes, which triggers astrocyte calcium signaling and releases ATP, which converts to adenosine, resulting in unstimulated synaptic depression [55], thus the CB1R activation facilitates LTP by inducing LTD [56].
Lateral synaptic regulation is also known to be involved in the striatum, where striatal astrocytes respond to the calcium mobilization by endocannabinoids released from the GABAergic medium-spiny neurons. CB1R stimulation activates glutaminergic (NMDA) pre-synaptic receptors, resulting in circuit-specific lateral synaptic potentiation [57]. The stimulation of CB1 receptors by endocannabinoids causes purine release in neurons in the amygdala area.
Neurons in the amygdala region are also activated by the CB1 receptor stimulation upon binding of the endocannabinoids, which leads to the purine release. The released purine binds to the adenosine receptors inducing synaptic regulation in distant neurons. Purines released from the astrocyte activation bind to the purinergic A1 receptors of the presynaptic terminals and also stimulate presynaptic A2A receptors. The endocannabinoids control synapses and modulate excitation/inhibition [45]. Long-term lateral potentiation is produced when astrocyte-to-pre-synapse communi-cation occurs simultaneously with retrograde nitric oxide release signaling. The endocannabinoids-induced astrocyte lateral modulation has been shown to contribute to synaptic plasticity [58].
Role of astrocytes in synaptic plasticity
The endocannabinoid system has been known to be associated with the long-term depression and long-term potentiation of the synaptic plasticity. Long-term potentiation is induced by astrocyte induced release of the neurotransmitter when endocannabinoids are activated simultaneously with postsynaptic activity [59]. This is due to a sustained increase in the short-term potentiation of cannabinoid astrocyte induced release of the neurotransmitter.
Certainly, the cannabinoid induced LTP requires the correlation of cannabinoid-induced glutamate signaling and nitric oxide release in the post synaptic transmission. The activation of presynaptic glutaminergic and protein kinase C is induced by the sequential coincidence of these signals, resulting in persistent potentiation for neurotransmitter release So, the long-term potentiation requires synchronized activity of the elements of the tripartite synapse, such as cannabinoid-induced calcium mobilization, which releases glutamate, nitric oxide generation, and protein C activation [54,59]. The endocannabinoids act as retrograde signals, depressing neurotransmission in the synapses and act as lateral messengers, inducing LTP in distant hetero-neuronal synapses via astrocyte network activation [59].
Induction of LTP requires the stimulation of CB1R by the endocannabinoids on the astrocyte membrane and induces the release of D-serine which is required for the formation of NMDA dependent LTP [22] and this plays important role in the formation of recognition memory.
LTD is an extensive synaptic plasticity phenomenon that involves several events with different underlying mechanisms. The induction of LTD may depend on NMDAR, mGluR, presynaptic A1 and A2 receptors or on retrograde signaling via presynaptic cannabinoid (CB1) receptors. By actively exchanging signals with synaptic components of neurons, astrocytes help to modulate Spike-Timing Dependent Plasticity (STDP). This induction approach can result in STDP, in which the direction tLTD (Timing-dependent Long-Term Depression) and magnitude of plasticity are determined by the order and interval of the two spikes [60].
Timing-dependent LTP is produced by Ca2+ influx through postsynaptic NMDA receptors, similar to LTP in the hippocampus and other synapses, at the L4-to-L2/3 (spiny stellate cells) synapse. tLTD, on the other hand, is more complicated, requiring the activation of postsynaptic metabotropic glutamate receptors (mGluRs), postsynaptic voltage gated Ca2+ channels, presynaptic NMDA receptors, and, most importantly, cannabinoid receptors, which are activated by endocannabinoids produced in the postsynaptic cell.
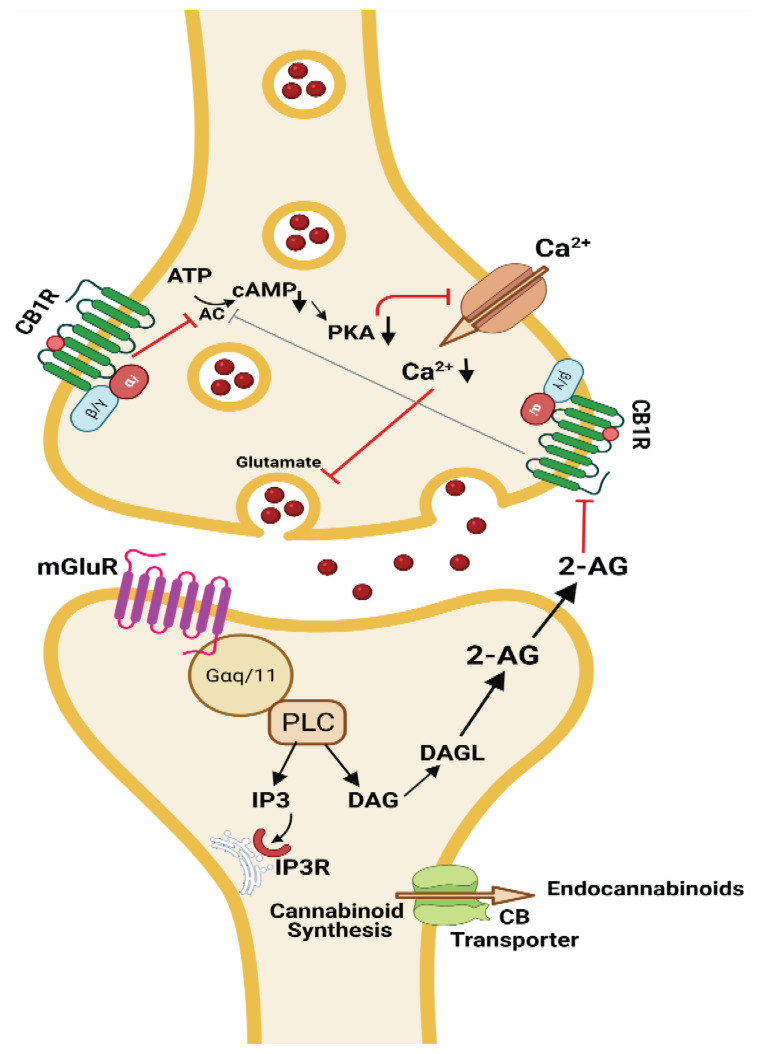
Min and Nevian employed patch clamp recording for the assessment of tLTD mechanisms in rodent barrel cortex [68]. They found that tLTD inducing CB receptors were located on astrocytes and their activation increases calcium spiking for the induction of tLTD. The recurrent activation of astroglial CB1Rs gradually elevated astrocyte Ca2+ levels and led to astrocytic glutamate mobilization, which activated presynaptic NMDA receptors, implying that astrocytes activation is adequate for the induction of synaptic depression. In this way they act as a memory buffer, integrating associated neuronal activity via calcium accumulation [61]. This endocannabinoid (CB1R) facilitated tLTD is vital for the sensory neocortex development. They also presented that cannabinoid receptor blockade or synthesis inhibition or loading with calcium chelators prevents the calcium spiking and eventually the induction of tLTD (Fig. 2). CB1R agonists alone do not cause long-term plasticity without concurrent presynaptic activity [62]. It was reported that when direct astrocytic stimulation is paired with presynaptic afferent activity, LTD is elicited. This is prevented by NMDA blockade [61] (Fig. 2).
Fig. 2.
Endocannabinoid signalling in neuronal communication and the mechanism involved in the induction of LTD. Arrows in black indicates facilitatory affect and red arrows indicates inhibitory actions. The image was created on Biorender.com under paid subscription.
Molecular interplay between endocannabinoid signaling in astrocytes and cognitive dysfunction in AD
Glial changes are often known to precede the formation of AD pathology [12]. In post-mortem brains of AD, reactive astrocytes are classically observed in region with high Aβ and tau pathology. Activation of microglia can also cause astrocyte reactivity [6]. Reactive astrocytes contribute to neuroinflammatory alterations in Alzheimer's disease by producing cytokines, inflammatory factors, nitric oxide (NO), and reactive oxygen species (ROS) and raising the redox imbalance. Astrocytes undergo structural and functional changes in AD [63,74], and the morphological changes arise in a region-specific pattern. Atrophy is first identified in the entorhinal cortex, followed by the prefrontal cortex, and finally the hippocampus at the pre-plaque stage [20].
Memory acquisition is sufficient to increase memory retrieval following pharmacogenetic or optogenetic activation of astroglial Gq signaling in the hippocampus, suggesting that astrocytes can improve cognition [64]. Astrocytic dysfunction may thus play a role in disease phenotype, but the extent to which it does so is uncertain. CB1Rs in astrocytic cells, not neuronal cells, were found to be key mediators of synaptic plasticity and behavioral effects in knock-out mouse models [46]. Despite the fact that there have been few studies on the cognitive and memory impairments in relation to astrocyte cannabinoids, it was reported that ablation of DAGL alters endocannabinoid concentrations and, eventually, behavior in a mouse model. It was demonstrated that heterosynaptic depression was increased by the CB1 agonist [65]. It was also reported that cannabis affected connexin 43 dysregulation via activating the CB1 receptor in astrocytes [66] and CB1 receptors also decreased glutamate release from glutaminergic neurons [67].
Recent research has shown that astrocytes respond to the endocannabinoids released during the induction process by elevating the levels of calcium and then releasing D-serine, which is required for LTP induction in vivo, as well as recognition memory consolidation [68,69,22]. In another study, it was reported that astroglial CB1R activation produced purines which induced LTP [70].
Anandamide reversed AMPA-induced neuroto-xicity in cultured astrocytes and down regulated GLT-1 and GLAST transporters in CB1 dependent pathway [71]. Similarly, THC protected against MDMA toxicity and prevented astrogliosis via CBR dependent-mechanism [72]. Besides these cannabinoids also exert anti-inflammatory activities that can mitigate neuroinflammation in AD and other neurodegenerative disorders.
Anandamide inhibited inflammation in astrocytes via CB1 cannabinoid receptors. UCM707, a selective anandamide (AEA) uptake inhibitor, inhibited the generation of proinflammatory mediators by LPS-stimulated astrocytes. UCM707 decreased NO release, iNOS expression, TNF-α and IL-1β levels, and increased IL-6 levels [51]. Another CB agonist, WIN55,212-2 inhibited their release of NO, TNF-α, in human cultured astrocytes [73].
Conclusions
Much research is required to elucidate the detailed mechanisms underlying the astrocyte cannabinoid system in Alzheimer’s disease. Astrocytes are promising therapeutic target because of their pathogenic potential, excitotoxicity-mediated neuronal death in AD caused by increasing extracellular glutamate levels, GLT1 loss, and GABA release from astrocytes. Pathological astrocyte remodelling that alters their homeostatic and neuroprotective roles and the development of glia-targeting therapeutics is still in its early stages, with the most difficult task being to find astrocyte specific and therapeutically relevant compounds. However, pharmacogenetic manipulation of astrocytes and astroglial glutamate uptake could be a way to mitigate excitotoxicity or another way is to target astrocyte genes.
Acknowledgements
The authors acknowledge DDT College of Medicine and University of Botswana, Gaborone, Botswana for their constant support.
Abbreviations
- A2A
Adenosine receptor
- Aβ
amyloid-beta
- AC
Adenylyl cyclase
- AD
Alzheimer's disease
- ATP
Adenosine triphosphate
- AEA
N-arachidonoyl ethanolamine/Anandamide
- AG
Arachidonyl glycerol
- AQP4
aquaporin4
- BACE
β-site amyloid precursor protein cleaving enzyme
- BDNF
brain derived neurotrophic factor
- CA1
cornu ammonis
- CB
Cannabinoid
- CBR
cannabinoid receptor
- CNS
central nervous system
- CCL5
C-C motif chemokine ligand 5
- DAGLα
diacylglycerol lipase α
- DSI
depolarization-induced suppression of inhibition
- DSE
depolarization-induced suppression of excitation
- EAAT
Excitatory amino acid transporters
- ECS
Endocannabinoid system
- GABA
gamma amino butyric acid
- GLUT1
glucose transporter1
- GLAST
glutamate aspartate transporter
- GWAS
genome wide association studies
- IGF1
Insulin-like growth factor
- IL
Interleukin
- iNOS
inducible nitic oxide synthase
- IP3
inositol 1,4,5-trisphosphate
- JNK
Jun N-terminal kinases
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- LTD
long-term depression
- MCP
monocyte chemoattractant protein
- MCT
monocarboxylate transporter
- MDMA
3,4-methylene dioxy-methamphetamine
- NMDA
N-methyl D-Aspartate
- P2Y6
purinoceptor
- ROS
reactive oxygen species
- THC
Tetrahydrocannabinol
- TNF α
Tumor necrosis factor alpha
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Rapaka D, Bitra VR, Challa SR, Adiukwu PC. mTOR signaling as a molecular target for the alleviation of Alzheimer’s disease pathogenesis. Neurochem Int. 2022;155:105311. doi: 10.1016/j.neuint.2022.105311. [DOI] [PubMed] [Google Scholar]
- 3.Scheltens P, Strooper BD, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer’s disease. Lancet. 2021;397:10284. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapaka D, Bitra VR, Challa SR, Adiukwu PC. Potentiation of microglial endocannabinoid signaling alleviates neuroinflammation in Alzheimer’s disease. Neuropeptides. 2021;90:102196. doi: 10.1016/j.npep.2021.102196. [DOI] [PubMed] [Google Scholar]
- 5.Fakhoury M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr Neuropharmacol. 2018;16:508–518. doi: 10.2174/1570159X15666170720095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol Med. 2019;25:77–95. doi: 10.1016/j.molmed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Frost GR, Li YM. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017;7:170228. doi: 10.1098/rsob.170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arranz AM, De Strooper B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 2019;18:406–414. doi: 10.1016/S1474-4422(18)30490-3. [DOI] [PubMed] [Google Scholar]
- 9.Pan J, Ma N, Yu B, Zhang W, Wan J. Transcriptomic profiling of microglia and astrocytes throughout aging. J Neuroinflammation. 2020;17:97. doi: 10.1186/s12974-020-01774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almad A, Maragakis NJ. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 2018;14:351–362. doi: 10.1038/s41582-018-0010-2. [DOI] [PubMed] [Google Scholar]
- 11.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ. Astrocytes in Alzheimer’s disease. Neurotherapeutics. 2010;7:399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V. Astroglia dynamics in ageing and Alzheimer’s disease. Curr Opin Pharmacol. 2016;26:74–79. doi: 10.1016/j.coph.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia. 2016;64:214–226. doi: 10.1002/glia.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 17.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 20.Verkhratsky A, Nedergaard M, Hertz L. Why are astrocytes important? Neurochem Res. 2015;40:389–401. doi: 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- 21.Fellin T. Communication between neurons and astrocytes:relevance to the modulation of synaptic and network activity. J Neurochem. 2009;108:533–544. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- 22.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–156. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 23.Barros LF, San Martín A, Ruminot I, Sandoval PY, Fernández-Moncada I, Baeza-Lehnert F, Arce-Molina R, et al. Near-critical GLUT1 and neurodegeneration. J Neurosci Res. 2017;95:2267–2274. doi: 10.1002/jnr.23998. [DOI] [PubMed] [Google Scholar]
- 24.Masaki K. Recent advances in understanding connexin gap junction pathology in demyelinating diseases. Clin Exp Neuroimmunol. 2020;11(Suppl 1):4–13. doi: 10.1111/cen3.12577. [DOI] [Google Scholar]
- 25.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Okamoto S, Dziewczapolski G, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110:E2518–E2527. doi: 10.1073/pnas.1313546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hefendehl JK, LeDue J, Ko RW, Mahler J, Murphy TH, MacVicar BA. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Commun. 2016;27:13441. doi: 10.1038/ncomms13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haughey NJ, Mattson MP. Alzheimer’s amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromolecular Med. 2003;3:173–180. doi: 10.1385/NMM:3:3:173. [DOI] [PubMed] [Google Scholar]
- 28.Simpson JE, Ince PG, Shaw PJ, Heath PR, Raman R, Garwood CJ. Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer’s pathology and APOE genotype. Neurobiol Aging. 2011;32:1795–1807. doi: 10.1016/j.neurobiolaging.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Guzman SJ, Gerevich Z. P2Y receptors in synaptic transmission and plasticity: therapeutic potential in cognitive dysfunction. Neural Plast. 2016;2016:1207393. doi: 10.1155/2016/1207393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceyzériat K, Ben Haim L, Denizot A, Pommier D, Matos M, Guillemaud O, Palomares MA, et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:104. doi: 10.1186/s40478-018-0606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, Halle A, Petzold GC. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol Med. 2019;11:e9665. doi: 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, Goebel UB, Weber JR. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998;22:295–305. doi: 10.1002/(SICI)1098-1136(199803)22:3<295::AID-GLIA8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Correa F, Ljunggren E, Mallard C, Nilsson M, Weber SG, Sandberg M. The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia: Involvement of p38 MAPK. Glia. 2011;59:785–799. doi: 10.1002/glia.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui M, Huang Y, Tian C, Zhao Y, Zheng J. FOXO3a inhibits TNF-α- and IL-1β-induced astrocyte proliferation: Implication for reactive astrogliosis. Glia. 2011;59:641–654. doi: 10.1002/glia.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metna-Laurent M, Marsicano G. Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia. 2015;63:353–364. doi: 10.1002/glia.22773. [DOI] [PubMed] [Google Scholar]
- 36.Specter S, Cabral G. Cannabinoids, immunity and resistance to infections. J Neuroimmunol. 1996;69:15–23. doi: 10.1016/0165-5728(96)00121-X. [DOI] [Google Scholar]
- 37.Howlett AC, Abood ME. CB1 and CB2 receptor pharmacology. Adv Pharmacol. 2017;80:169–206. doi: 10.1016/bs.apha.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurman LD, Lu D, Kendall DA, Howlett AC, Lichtman AH. Molecular mechanism and cannabinoid pharmacology. Handb Exp Pharmacol. 2020;258:323–353. doi: 10.1007/164_2019_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaseghi S, Nasehi M, Zarrindast M-Reza. How do stupendous cannabinoids modulate memory processing via affecting neurotransmitter systems? Neurosci Biobehav Rev. 2021;120:173–221. doi: 10.1016/j.neubiorev.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Araujo DJ, Tjoa K, Saijo K. The endocannabinoid system as a window into micoglial biology and its relationship to autism. Front Cell Neurosci. 2019;13:424. doi: 10.3389/fncel.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffy SS, Hayes JP, Fiore NT, Moalem Taylor G. The cannabinoid system and microglia in health and disease. Neuropharmacology. 2021;190:108555. doi: 10.1016/j.neuropharm.2021.108555. [DOI] [PubMed] [Google Scholar]
- 42.Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elangovan S, Holsinger RMD. Cyclical amyloid beta-astrocyte activity induces oxidative stress in Alzheimer’s disease. Biochimie. 2020;171–172:38–42. doi: 10.1016/j.biochi.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Alasmari F, Alshammari MA, Alasmari AF, Alanazi WA, Alhazzani K. Neuroinflammatory cytokines induce amyloid beta neurotoxicity through modulating amyloid precursor protein levels/metabolism. Biomed Res Int. 2018;2018:3087475. doi: 10.1155/2018/3087475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 47.Hegyi Z, Kis G, Hollo K, Ledent C, Antal M. Neuronal and glial localization of the cannabinoid-1 receptor in the superficial spinal dorsal horn of the rodent spinal cord. Eur J Neurosci. 2009;30:251–262. doi: 10.1111/j.1460-9568.2009.06816.x. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez-Rodriguez A, Bonilla-Del Rio I, Puente N, Gomez-Urquijo SM, Fontaine CJ, Egana-Huguet J, Elezgarai I, et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia. 2018;66:1417–1431. doi: 10.1002/glia.23314. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Covelo A, Eraso-Pichot A, Fernández-Moncada I, Serrat R, Marsicano G. CB1R-dependent regulation of astrocyte physiology and astrocyte-neuron interactions. Neuropharmacology. 2021;195:108678. doi: 10.1016/j.neuropharm.2021.108678. [DOI] [PubMed] [Google Scholar]
- 53.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 54.Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Szabó GG, Lenkey N, Holderith N, Andrási T, Nusser Z, Hájos N. Presynaptic calcium channel inhibition underlies CB1 cannabinoid receptormediated suppression of GABA release. J Neurosci. 2014;34:7958–7963. doi: 10.1523/JNEUROSCI.0247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 57.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive d-serine release. J Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega-Gutiérrez S, Molina-Holgado E, Guaza C. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia. 2005;52:163–168. doi: 10.1002/glia.20229. [DOI] [PubMed] [Google Scholar]
- 59.Pacholko AG, Wotton CA, Bekar LK. Astrocytes-the ultimate effectors of long-range neuromodulatory networks? Front Cell Neurosci. 2020;14:581075. doi: 10.3389/fncel.2020.581075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verkhratsky A, Chvatal A. NMDA receptors in astrocytes. Neurochem Res. 2020;45:122–133. doi: 10.1007/s11064-019-02750-3. [DOI] [PubMed] [Google Scholar]
- 61.Covelo A, Araque A. Lateral regulation of synaptic transmission by astrocytes. Neuroscience. 2016;323:62–66. doi: 10.1016/j.neuroscience.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 62.Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol. 2007;585:843–852. doi: 10.1113/jphysiol.2007.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- 64.Martín R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349:730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- 65.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 66.Gómez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martín-Fernández M, Shigemoto R, Luján R, Araque A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb Cortex. 2015;25:3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- 67.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min R, Nevian T. Astrocyte signaling controls spike timing dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 69.Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/S0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 70.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018;174:59–71.e14. doi: 10.1016/j.cell.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Schurman LD, Carper MC, Moncayo LV, Ogasawara D, Richardson K, Yu L. Diacylglycerol lipase-alpha regulates hippocampal-dependent learning and memory processes in mice. J Neurosci. 2019;39:5949–5965. doi: 10.1523/JNEUROSCI.1353-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Froger N, Orellana JA, Cohen-Salmon M, Ezan P, Amigou E, Saez JC, Giaume C. Cannabinoids prevent the opposite regulation of astroglial connexin43 hemichannels and gap junction channels induced by proinflammatory treatments. J Neurochem. 2009;111:1383–1397. doi: 10.1111/j.1471-4159.2009.06407.x. [DOI] [PubMed] [Google Scholar]
- 74.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 75.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, Bellocchio L, et al. Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron. 2018;98:935–944. doi: 10.1016/j.neuron.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 77.Rasooli-Nejad S, Palygin O, Lalo U, Pankratov Y. Cannabinoid receptors contribute to astroglial Ca2+-signalling and control of synaptic plasticity in the neocortex. Philos Trans R Soc Lond B Biol Sci. 2014;369:20140077. doi: 10.1098/rstb.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lorıa F, Petrosino S, Hernangomez M, Mestre L, Spagnolo A, Correa F, Di Marzo V, Docagne F, Guaza C. An endocannabinoid tone limits excitotoxicity in vitro and in a model of multiple sclerosis. Neurobiol Dis. 2010;37:166–176. doi: 10.1016/j.nbd.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 79.Tourino C, Zimmer A, Valverde O. THC prevents MDMA neurotoxicity in mice. PLoS One. 2010;5:e9143. doi: 10.1371/journal.pone.0009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 81.Rapaka D, Bitra VR, Ummidi R, Akula A. Benincasa hispida alleviates amyloid pathology by inhibition of Keap1/Nrf2-axis: Emphasis on oxidative and inflammatory stress involved in Alzheimer’s disease model. Neuropeptides. 2021;88:102151. doi: 10.1016/j.npep.2021.102151. [DOI] [PubMed] [Google Scholar]