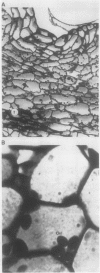
Abstract
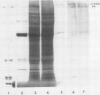
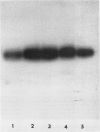
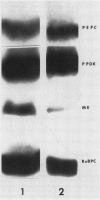
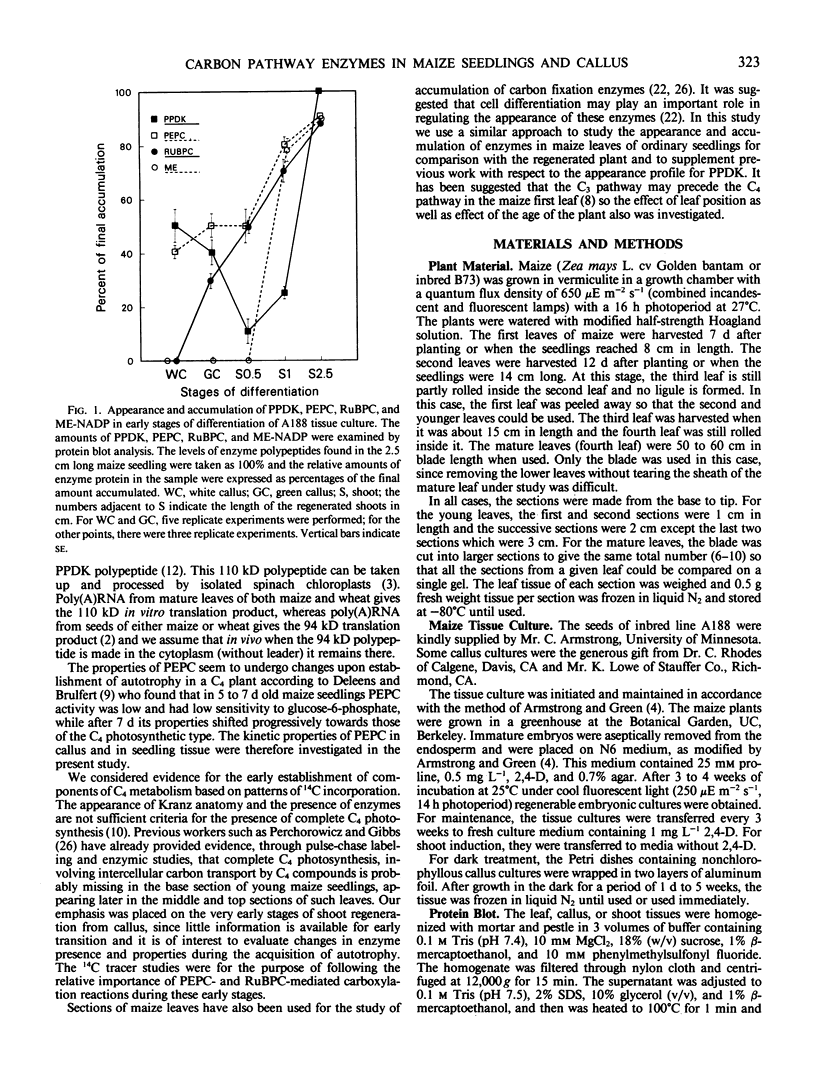
Regenerating maize A188 tissue cultures were examined for the presence of enzymes involved in C4 photosynthesis, for cell morphology, and for 14C labeling kinetics to study the implementation of this pathway during plant development. For comparison, sections of maize seedling leaves were examined. Protein blot analysis using antibodies to leaf enzymes showed a different profile of these enzymes during the early stages of shoot regeneration from callus from the closely-coordinated profile observed in seedling leaves. Pyruvate orthophosphate dikinase (PPDK) (EC 2.7.9.1) and phosphoenolpyruvate carboxylase (PEPC) (EC 4.1.1.31) were found in nonchlorophyllous callus while ribulose 1,5-bisphosphate carboxylase (RuBPC, EC 4.1.1.39) and malic enzyme, NADP-specific (ME-NADP) (EC 1.3.1.37) were not detectable until later.
Enzyme activity assays showed the presence of ME-NADP as well as PEPC and PPDK in nonchlorophyllous callus. However, the activities of ME-NADP and PEPC had properties similar to those of the enzymes from C3 leaves and from etiolated C4 leaf tissues, but differing from the corresponding enzymes in the mature leaf.
Immunoprecipitation of in vitro translation products of poly(A)RNA extracted from embryoid-forming callus showed both the 110 kilodalton precursor to chloroplast PPDK and the 94 kilodalton polypeptide. Therefore, the chloroplast tye of PPDK mRNA is present prior to the appearance of leaf morphology.
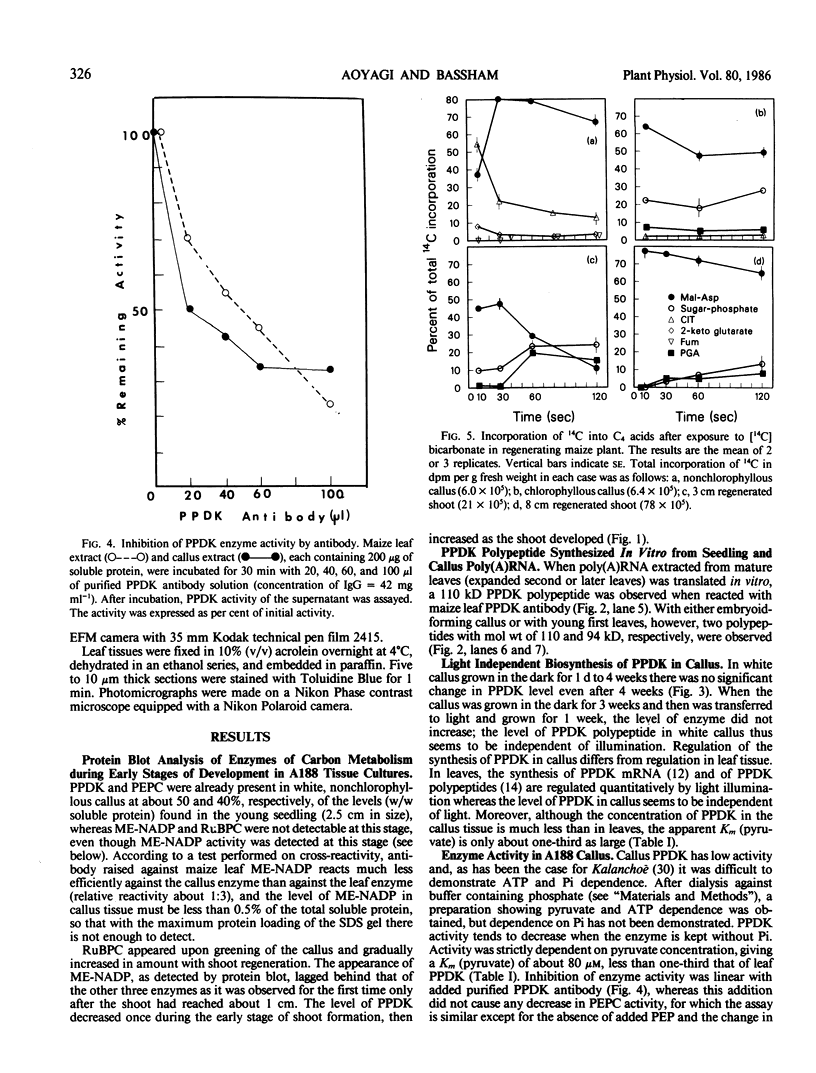
Analysis of the labeled products of 14CO2 fixation by nonchlorophyllous calli indicated β-carboxylation to give acids of the tricarboxylic acid cycle, but no incorporation into phosphoglycerate. With greening of the callus, some incorporation into phosphoglycerate and sugar phosphates occurred, and this increased in shoots as they developed, although with older shoots the increase in β-carboxylation products was even greater. Analysis of enzyme levels in young leaf sections by protein blot and of 14C-labeling patterns in the present study are in general agreement with enzyme activity determinations of previous studies, providing additional information about PPDK levels, and supporting the model proposed for developing young leaves.
These results suggest that maize leaves begin to express C4 enzymes during ontogeny through several stages from greening and cell differentiation as seen in the callus and then shoot formation, and finally acquire capacity for full C4 photosynthesis during leaf development concomitant with the development of Kranz anatomy and accumulation of large amounts of enzymes involved in carbon metabolism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase mRNA organ specificity in wheat and maize. Plant Physiol. 1984 Sep;76(1):278–280. doi: 10.1104/pp.76.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase of c(3) seeds and leaves as compared to the enzyme from maize. Plant Physiol. 1984 Jun;75(2):387–392. doi: 10.1104/pp.75.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hague D. R., Uhler M., Collins P. D. Cloning of cDNA for pyruvate, Pi dikinase from maize leaves. Nucleic Acids Res. 1983 Jul 25;11(14):4853–4865. doi: 10.1093/nar/11.14.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Association of NADP- and NAD-linked malic enzyme acitivities in Zea mays: relation to C4 pathway photosynthesis. Arch Biochem Biophys. 1977 Mar;179(2):361–369. doi: 10.1016/0003-9861(77)90123-0. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Kennedy R. A., Barnes J. E. Photosynthesis in c(4) plant tissue cultures: significance of kranz anatomy to c(4) Acid metabolism in c(4) plants. Plant Physiol. 1977 Apr;59(4):600–603. doi: 10.1104/pp.59.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. A. Photorespiration in c(3) and c(4) plant tissue cultures: significance of kranz anatomy to low photorespiration in c(4) plants. Plant Physiol. 1976 Oct;58(4):573–575. doi: 10.1104/pp.58.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Sinha S. K. Change in the predominance from C 4 to C 3 pathway following anthesis in sorghum. Biochem Biophys Res Commun. 1973 May 1;52(1):121–124. doi: 10.1016/0006-291x(73)90962-5. [DOI] [PubMed] [Google Scholar]
- Laetsch W. M., Kortschak H. P. Chloroplast structure and function in tissue cultures of a c(4) plant. Plant Physiol. 1972 Jun;49(6):1021–1023. doi: 10.1104/pp.49.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Gibbs M. Carbon dioxide fixation and related properties in sections of the developing green maize leaf. Plant Physiol. 1980 May;65(5):802–809. doi: 10.1104/pp.65.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Occurrence of Pyruvate Orthophosphate Dikinase in the Succulent Plant, Kalanchoë daigremontiana Hamet. et. Perr. Plant Physiol. 1975 Nov;56(5):605–607. doi: 10.1104/pp.56.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]