Abstract
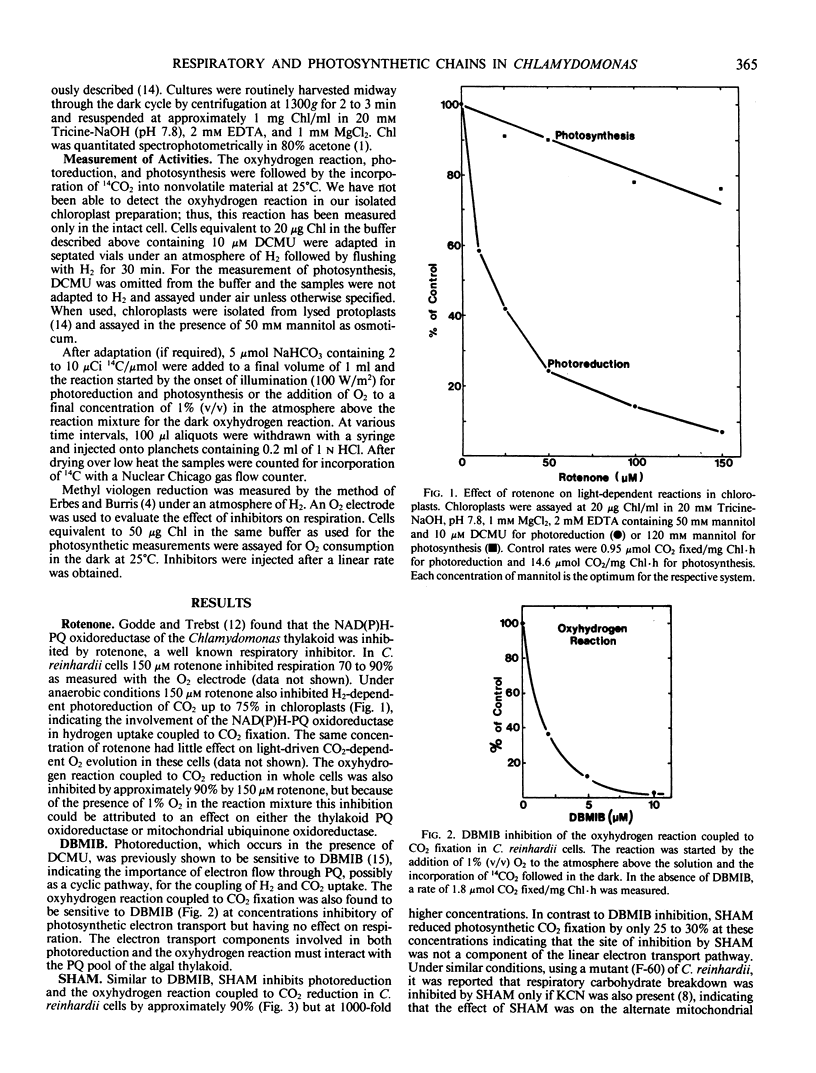
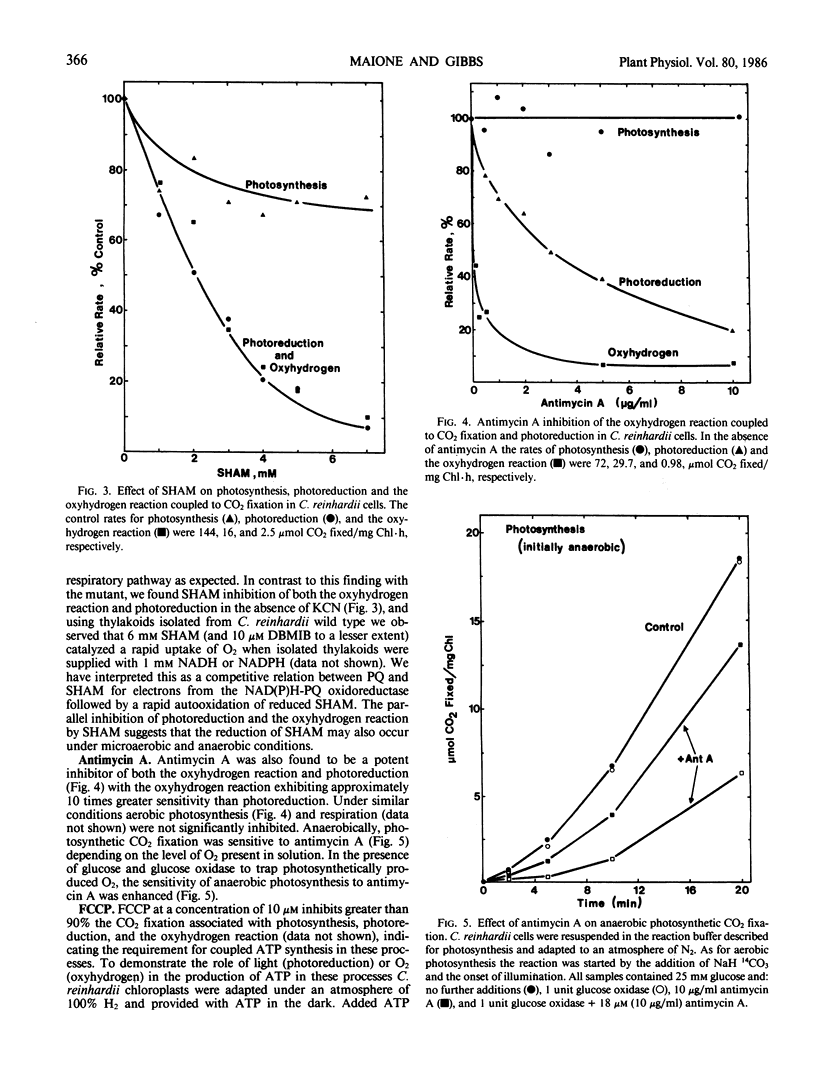
The hydrogenase-dependent processes, photoreduction and the dark oxyhydrogen reaction, both of which can support CO2 assimilation, were compared with aerobic photosynthesis and respiration for their sensitivity to electron transport inhibitors in cells and intact chloroplasts of Chlamydomonas reinhardii 11-32/6. Photoreduction but not photosynthesis was inhibited in chloroplasts and the oxyhydrogen reaction detected only in cells was inhibited up to 75 and 90%, respectively, by 150 micromolar rotenone, indicating the involvement of a NAD(P)H-plastoquinone oxidoreductase in the hydrogen utilizing pathways. The oxyhydrogen reaction coupled to CO2 fixation was inhibited more than 95% by 10 micromolar 2,5 - dibromo - 3 - methyl - 6 - isopropyl - p - benzoquinone (DBMIB), a concentration which did not affect respiratory activity. In cells, both photoreduction and the oxyhydrogen reaction exhibited a similar sensitivity to salicylhydroxamic acid (SHAM) showing approximately 90% inhibition by 7 millimolar concentration. Photosynthesis was inhibited only 30% by the same concentration of SHAM. Antimycin A (18 micromolar, 10 micrograms per milliliter) inhibited both photoreduction (80%) and the oxyhydrogen reaction (92%) in cells with the oxyhydrogen reaction being approximately 10 times more sensitive to lower concentrations of the inhibitor. Antimycin A at 18 micromolar concentration did not inhibit photosynthetic CO2 fixation unless the cells were adapted to an atmosphere of N2 and the reaction conducted anaerobically. Photosynthesis, photoreduction, and the oxyhydrogen reaction coupled to CO2 fixation were all inhibited greater than 90% by 10 micromolar carbonylcyanide-p-trifluoromethoxyphenylhydrazone. ATP added to chloroplasts adapted to an atmosphere of H2 could support CO2 uptake in the dark. These results are interpreted as evidence that photoreduction and the oxyhydrogen reaction involve some common components of thylakoidal electron transport pathways in Chlamydomonas including NAD(P)H-plastoquinone oxidoreductase and the plastoquinone pool. An O2-consuming thylakoidal or mitochondrial reaction is an additional component of the oxyhydrogen reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., Gibbs M. Kinetics of the Oxyhydrogen Reaction in the Presence and Absence of Carbon Dioxide in Scenedesmus obliquus. Plant Physiol. 1981 Jan;67(1):129–132. doi: 10.1104/pp.67.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller R. P., Gibbs M. Fermentative Metabolism of Chlamydomonas reinhardtii: II. Role of Plastoquinone. Plant Physiol. 1985 Feb;77(2):509–511. doi: 10.1104/pp.77.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Nishimura M., Chance B. Energy and Electron Transfer Systems of Chlamydomonas reinhardi. I. Photosynthetic and Respiratory Cytochrome Systems of the Pale Green Mutant. Plant Physiol. 1969 Apr;44(4):527–534. doi: 10.1104/pp.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione T. E., Gibbs M. Hydrogenase-Mediated Activities in Isolated Chloroplasts of Chlamydomonas reinhardii. Plant Physiol. 1986 Feb;80(2):360–363. doi: 10.1104/pp.80.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Evidence for the participation of the reductive pentose phosphate cycle in photoreduction and the oxyhydrogen reaction. Plant Physiol. 1968 Apr;43(4):649–652. doi: 10.1104/pp.43.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]


