Abstract
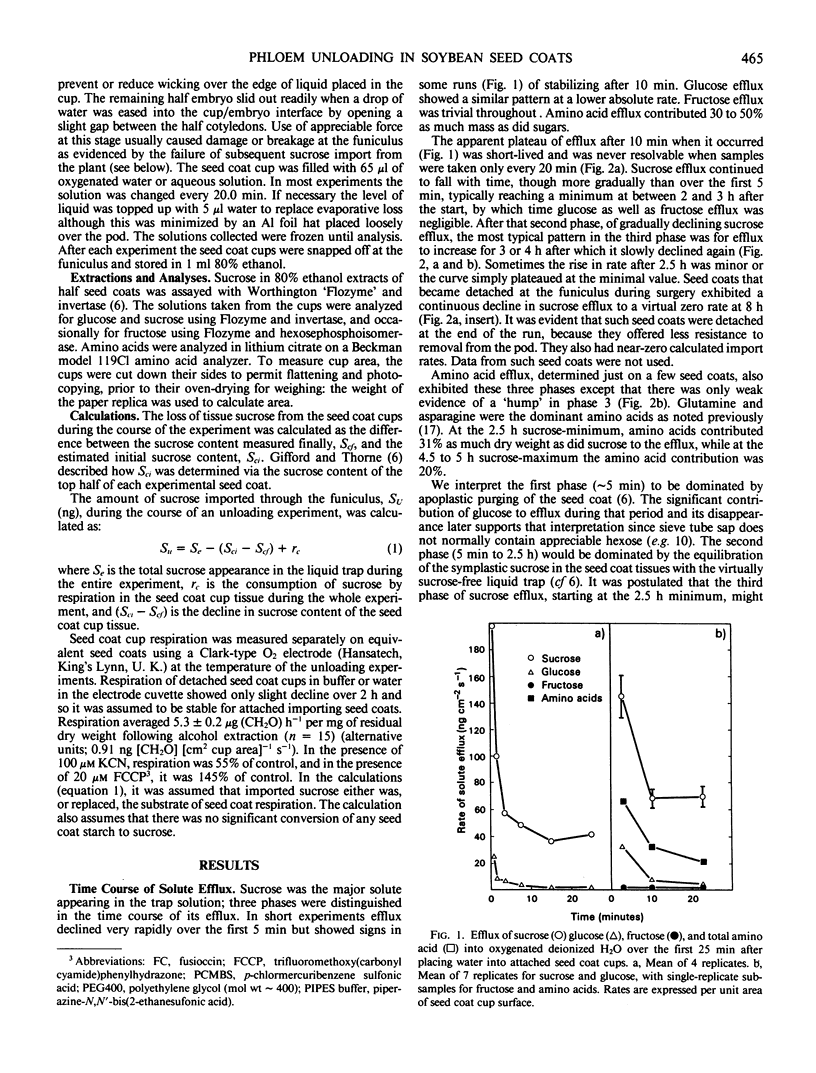
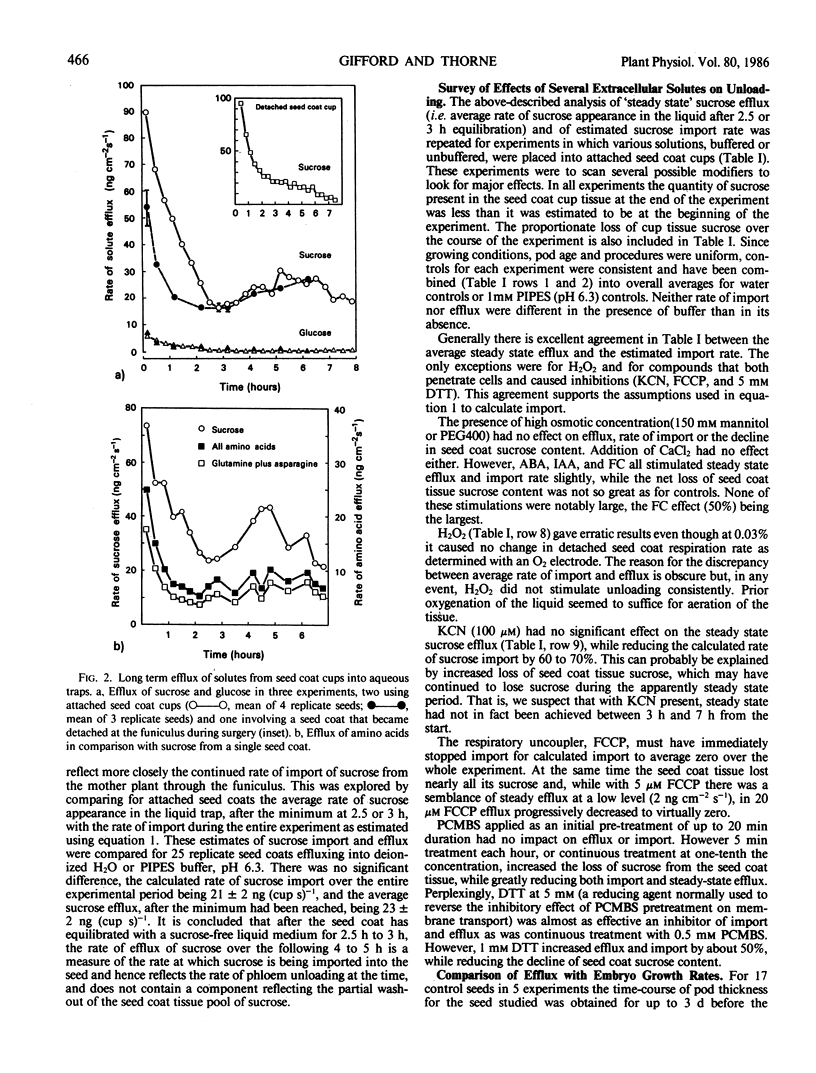
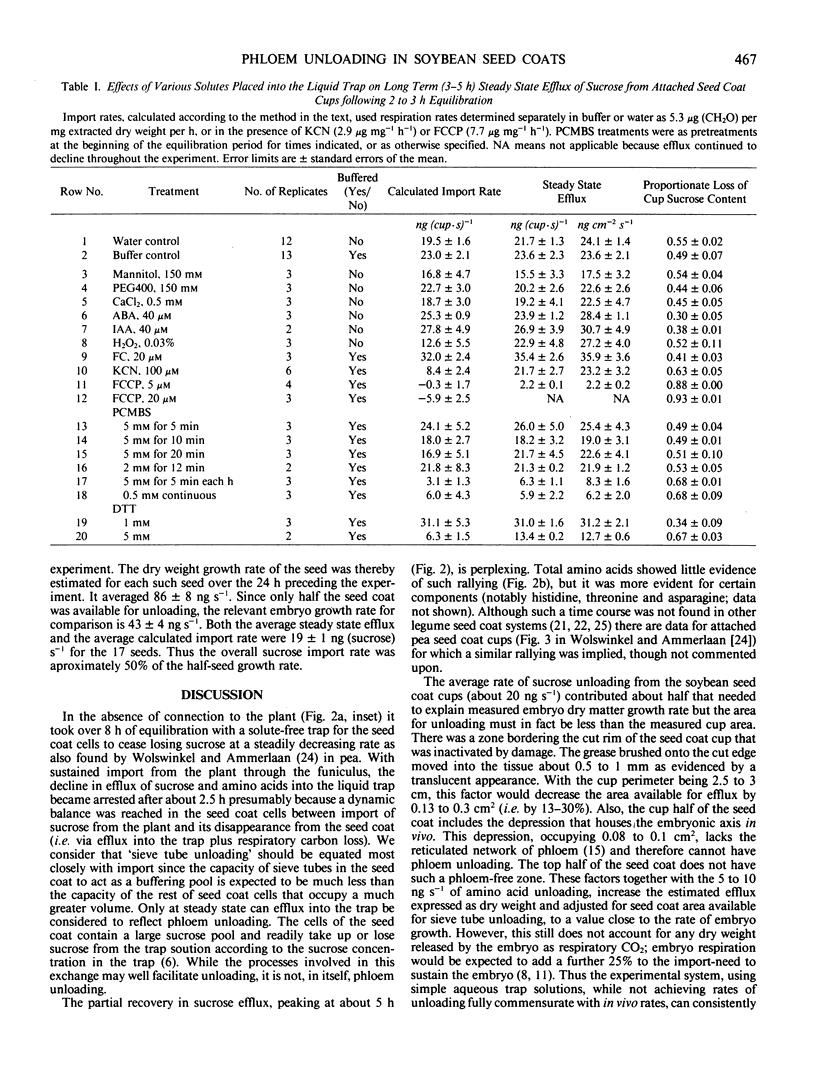
The time-course of sucrose efflux from attached seedcoats (having their embryos surgically removed) into aqueous traps placed in the `empty ovules' had three phases. The first phase lasted 10 minutes and probably was a period of apoplastic flushing. The second lasted 2 to 3 hours and is thought to be a phase of equilibration of seed coat symplast with the frequently refreshed liquid. The third phase of relatively steady efflux was postulated to reflect the continued import of sucrose from the plant, and hence to reflect the rate of sieve tube unloading. The average steady state efflux was equal under most conditions to the estimated rate of sucrose import. Efflux and import were unaffected by 150 millimolar osmoticum (mannitol or polyethylene glycol [molecular weight about 400]), by 0.5 millimolar CaCl2, or by pretreatments up to 20 minutes with p-chloromercuribenzenesulfonic acid (PCMBS); they were enhanced by 40 micromolar abscisic acid, 40 micromolar indoleacetic acid, 20 micromolar fusicoccin, and 1 millimolar dithiothreitol (DTT) and were inhibited by 100 micromolar KCN, by 0.03% H2O2, by 20 micromolar and 5 micromolar trifluoromethoxy (carbonyl cyamide) phenylhydrazone, by repeated 5 minutes per hour treatments with 5 millimolar PCMBS, and by 5 millimolar DTT. The `steady state' sucrose efflux was able to account for about half the rate of dry weight growth of the embryo, but stabilization of the system with <1 millimolar DTT taken together with other considerations is likely to give good correspondence between experimental unloading rates and in vivo growth rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giaquinta R. Mechanism of cyanide inhibition of Phloem translocation. Plant Physiol. 1977 Feb;59(2):178–180. doi: 10.1104/pp.59.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. M., Thorne J. H. Sucrose Concentration at the Apoplastic Interface between Seed Coat and Cotyledons of Developing Soybean Seeds. Plant Physiol. 1985 Apr;77(4):863–868. doi: 10.1104/pp.77.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Peoples M. B., Atkins C. A. Spontaneous Phloem bleeding from cryopunctured fruits of a ureide-producing legume. Plant Physiol. 1984 Mar;74(3):499–505. doi: 10.1104/pp.74.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples M. B., Pate J. S., Atkins C. A., Murray D. R. Economy of water, carbon, and nitrogen in the developing cowpea fruit. Plant Physiol. 1985 Jan;77(1):142–147. doi: 10.1104/pp.77.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler J. R., Brenner M. L., Brun W. A. Abscisic Acid and its relationship to seed filling in soybeans. Plant Physiol. 1984 Oct;76(2):301–306. doi: 10.1104/pp.76.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Morphology and ultrastructure of maternal seed tissues of soybean in relation to the import of photosynthate. Plant Physiol. 1981 May;67(5):1016–1025. doi: 10.1104/pp.67.5.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Rainbird R. M. An in vivo technique for the study of Phloem unloading in seed coats of developing soybean seeds. Plant Physiol. 1983 May;72(1):268–271. doi: 10.1104/pp.72.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


